
(8��)�ס������صĵ缫���϶���������̼������ش��������⣺
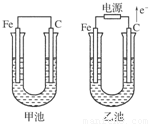
(1)�������о�ΪCuSO4��Һ����Ӧһ��ʱ���
���к�ɫ�����������Ǽ׳��е�________�����ҳ��е�________����
���ҳ��������ĵ缫��Ӧʽ��_______________________________________________��
(2)�������о�Ϊ����NaCl��Һ��
��д���ҳ����ܷ�Ӧ�����ӷ���ʽ________________________________________��
�ڼ׳���̿���ϵĵ缫��Ӧʽ��________���ҳ���̿���ϵĵ缫��Ӧ����________(�������Ӧ����ԭ��Ӧ��)��
�۽�ʪ��ĵ���KI��ֽ�����ҳ�̿��������������ֽ��������һ��ʱ����ַ�����ɫ��ȥ��������Ϊ������Cl2�����ɵ�I2������������Ӧ��Cl2��I2���ʵ���֮��Ϊ5 ��1�������������ᣬ�÷�Ӧ�Ļ�ѧ����ʽΪ
________________________________________________________________________��
�����ҳ�ת��0.02mol e����ֹͣʵ�飬������Һ�����200mL������Һ���Ⱥ��pH��________��
 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(8��)�ס������صĵ缫���϶���������̼������ش��������⣺
(1)�������о�ΪCuSO4��Һ����Ӧһ��ʱ���
���к�ɫ�����������Ǽ׳��е�________�����ҳ��е�________����
���ҳ��������ĵ缫��Ӧʽ��_______________________________________________��
(2)�������о�Ϊ����NaCl��Һ��
��д���ҳ����ܷ�Ӧ�����ӷ���ʽ________________________________________��
�ڼ׳���̿���ϵĵ缫��Ӧʽ��________���ҳ���̿���ϵĵ缫��Ӧ����________(�������Ӧ����ԭ��Ӧ��)��
�۽�ʪ��ĵ���KI��ֽ�����ҳ�̿��������������ֽ��������һ��ʱ����ַ�����ɫ��ȥ��������Ϊ������Cl2�����ɵ�I2������������Ӧ��Cl2��I2���ʵ���֮��Ϊ5��1�������������ᣬ�÷�Ӧ�Ļ�ѧ����ʽΪ
________________________________________________________________________��
�����ҳ�ת��0.02mol e����ֹͣʵ�飬������Һ�����200mL������Һ���Ⱥ��pH��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��߿���ѧһ�ָ�ϰר���ۺϲ���6����ѧ��Ӧ�������仯���ս̰棩 ���ͣ������
(8��)�ס������صĵ缫���϶���������̼������ش��������⣺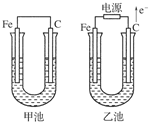
(1)�������о�ΪCuSO4��Һ����Ӧһ��ʱ���
���к�ɫ�����������Ǽ׳��е�________�����ҳ��е�________����
���ҳ��������ĵ缫��Ӧʽ��_______________________________________________��
(2)�������о�Ϊ����NaCl��Һ��
��д���ҳ����ܷ�Ӧ�����ӷ���ʽ________________________________________��
�ڼ׳���̿���ϵĵ缫��Ӧʽ��________���ҳ���̿���ϵĵ缫��Ӧ����________(�������Ӧ����ԭ��Ӧ��)��
�۽�ʪ��ĵ���KI��ֽ�����ҳ�̿��������������ֽ��������һ��ʱ����ַ�����ɫ��ȥ��������Ϊ������Cl2�����ɵ�I2������������Ӧ��Cl2��I2���ʵ���֮��Ϊ5 ��1�������������ᣬ�÷�Ӧ�Ļ�ѧ����ʽΪ
________________________________________________________________________��
�����ҳ�ת��0.02mol e����ֹͣʵ�飬������Һ�����200mL������Һ���Ⱥ��pH��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�������ʡ�����и߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
�������֣���ͼ Ϊ������ļ����������أ���ش�
Ϊ������ļ����������أ���ش�
((1)�׳���Ϊ�õ��ԭ������ͭ��װ�ã�A����___��������____���缫��ӦΪ________��B����_________��������__________����Ҫ�缫��ӦΪ___________���������ҺΪ_____________��
(2)�ҳ���������������̪��Һ����ʼһ��ʱ���____�������ʺ�ɫ��
(3)���ײ���������12.8 g�����Ҳ������ų������ڱ�״���µ����Ϊ__ ______��
______��
(4)���������Ҳ�ʣ��Һ��Ϊ400 mL�������õ���Һ�����ʵ���Ũ��Ϊ__________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com