
�л���W(C3H6O3)����NaHCO3��Ӧ�������к������ֻ�Բ�ͬ����ԭ�ӣ�������Ϊ3��1��1��1��
��1��W�Ľṹ��ʽ������������������������
��2��W�Ĺ�ҵ�ϳ�·������ͼ��ʾ��

��֪����.A��B��C��D��W�����к�����̼ͬԭ������
��.![]()
��д��A�Ľṹ��ʽ������������������������
��B������Cu(OH)2��Ӧ�Ļ�ѧ����ʽ������������������������
��D��NaOHˮ��Һ�з�Ӧ�Ļ�ѧ����ʽ������������������
��C��ͬ���칹���У��ܹ���Ag(NH3)2OH��Һ��Ӧ�������������֡�
��3����ҵ��Ҳ��������ͼ��ʾ�ϳ�·������W��
![]()
��֪��![]()
���ںϳ�·�߿�ͼ��������Ӧ�л���Ľṹ��ʽ��
��4��W��һ�������£���Ӧ���ɱ�����(C6H8O4)(����Ԫ���ṹ)���÷�Ӧ�Ļ�ѧ����ʽ��(�л���д�ṹ��ʽ) ��
��������һ�������¾ۺ����ɾ۱�����(![]() )���۱���������˿���Ƴ���������ߣ��������ڿ��Զ���������ΪW���۱����������ڽ���Ļ�ѧ����ʽ��
)���۱���������˿���Ƴ���������ߣ��������ڿ��Զ���������ΪW���۱����������ڽ���Ļ�ѧ����ʽ��
��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��20�֣��л���W(C3H6O3)����NaHCO3��Ӧ�������к������ֻ�Բ�ͬ����ԭ�ӣ�������Ϊ3��1��1��1��
��1��W�Ľṹ��ʽ������������������������
��2��W�Ĺ�ҵ�ϳ�·������ͼ��ʾ��
��֪����.A��B��C��D��W�����к�����̼ͬԭ������
��.
��д��A�Ľṹ��ʽ������������������������
��B������Cu(OH)2��Ӧ�Ļ�ѧ����ʽ������������������������
��D��NaOHˮ��Һ�з�Ӧ�Ļ�ѧ����ʽ������������������
��C��ͬ���칹���У��ܹ���Ag(NH3)2OH��Һ��Ӧ�������������֡�
��3����ҵ��Ҳ��������ͼ��ʾ�ϳ�·������W��
��֪��
���ںϳ�·�߿�ͼ��������Ӧ�л���Ľṹ��ʽ��
��4��W��һ�������£���Ӧ���ɱ�����(C6H8O4)(����Ԫ���ṹ)���÷�Ӧ�Ļ�ѧ����ʽ��(�л���д�ṹ��ʽ) ��
��������һ�������¾ۺ����ɾ۱�����()���۱���������˿���Ƴ���������ߣ��������ڿ��Զ���������ΪW���۱����������ڽ���Ļ�ѧ����ʽ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ɽ��ʡ�߶���ѧ����ĩ���Ի�ѧ���� ���ͣ������
��20�֣��л���W(C3H6O3)����NaHCO3��Ӧ�������к������ֻ�Բ�ͬ����ԭ�ӣ�������Ϊ3��1��1��1��
��1��W�Ľṹ��ʽ������������������������
��2��W�Ĺ�ҵ�ϳ�·������ͼ��ʾ��

��֪����.A��B��C��D��W�����к�����̼ͬԭ������
��.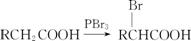
��д��A�Ľṹ��ʽ������������������������
��B������Cu(OH)2��Ӧ�Ļ�ѧ����ʽ������������������������
��D��NaOHˮ��Һ�з�Ӧ�Ļ�ѧ����ʽ������������������
��C��ͬ���칹���У��ܹ���Ag(NH3)2OH��Һ��Ӧ�������������֡�
��3����ҵ��Ҳ��������ͼ��ʾ�ϳ�·������W��

��֪��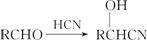
���ںϳ�·�߿�ͼ��������Ӧ�л���Ľṹ��ʽ��
��4��W��һ�������£���Ӧ���ɱ�����(C6H8O4)(����Ԫ���ṹ)���÷�Ӧ�Ļ�ѧ����ʽ��(�л���д�ṹ��ʽ) ��
��������һ�������¾ۺ����ɾ۱�����(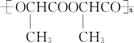 )���۱���������˿���Ƴ���������ߣ��������ڿ��Զ���������ΪW���۱����������ڽ���Ļ�ѧ����ʽ��
)���۱���������˿���Ƴ���������ߣ��������ڿ��Զ���������ΪW���۱����������ڽ���Ļ�ѧ����ʽ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��0115 ��ĩ�� ���ͣ������
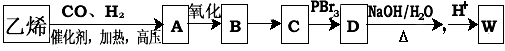
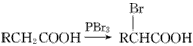
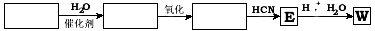
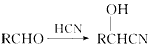
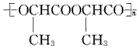 )���۱���������˿���Ƴ���������ߣ��������ڿ��Զ���������ΪW���۱����������ڽ���Ļ�ѧ����ʽ��
)���۱���������˿���Ƴ���������ߣ��������ڿ��Զ���������ΪW���۱����������ڽ���Ļ�ѧ����ʽ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(7��)ԭ�Ӻ˴Ź����ף�PMR�����о��л�������ṹ����Ҫ����֮һ�������о��Ļ���������У�����λ����ȫ��ͬ����ԭ�ӣ�����Hԭ�ӣ��ں˴Ź������г���ͬһ���źŷ壬���з��ǿ�������Hԭ�ӵ���Ŀ�����ȡ��� ��ȩ��CH3CHO���ں˴Ź���������2���źŷ壬��ǿ��֮��Ϊ3:1��
(1)CH3CH2COOH��PMR���й۲쵽���ַ��ǿ��֮��Ϊ ��
(2)ʵ���п��Ը���PMR���й۲쵽����ԭ�Ӹ����ķ�ֵ�����ȷ���л���Ľṹ����֪�л���W(C3H6O3)����NaHCO3��Ӧ���ҷ�ֵǿ��֮��Ϊ3:1:1:1�����ƶϳ����Ӧ�Ľṹ��ʽΪ ��
(3)��֪�л���A��W�����Ժ��ֱ�����ϣ�ֻҪ����һ������ȫȼ�պ������ˮ������Ҳһ����
�ٷ�����������Է���������С���л���A�� (��д�ṹ��ʽ)��
����A���������Է���������ȣ���������Na2CO3��Ӧ�ų�CO2��1mol A���������Ʒ�Ӧ�ɲ���22.4L H2(��״��)����A�Ľṹ��ʽΪ �� ��
(4)W��������;������ȡ��
![]()
W
д����Ӧ�ڷ������������ �� д��C�Ľṹ��ʽ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com