
��1����A��F��
A��11H��21H
B������ͼ���
C�����ʯ��ʯī
D����������춡��
E��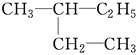 ��
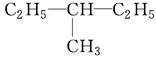
��
F��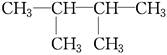 ��
��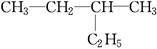
������ͬ���칹�����________��������ͬλ�ص���________��������ͬ�����������________��������ͬһ�����ʵ���________��
��2����ȫȼ��0.1 molij����ȼ�ղ�������ͨ��Ũ���ᡢŨ��Һ��ʵ������Ƶ�Ũ��������9 g��Ũ��Һ����17.6 g�������Ļ�ѧʽ______����д�������п��ܵĽṹ��ʽ__________________��
��11�֣���1����D��F����A����C����E
��2��C4H10��CH3��CH2��CH2��CH3��
��������
�����������1������ͬλ�ء�ͬ���칹�����ͬ���칹�塢ͬ��������ĸ�����⡣
��2����װ������Ϊˮ����������װ������Ϊ������̼��������
��n��H2O��=9.0g��18g/mol=0.5mol��n��H��=1mol��
��1mol���к���10molHԭ�ӣ�
n��CO2��=17.6g��44g/mol=0.4mol��n��C��=0.4mol��
��1mol���к���4molCԭ�ӣ�
���Ը����ķ���ʽΪC4H10��
�𣺸����Ļ�ѧʽΪC4H10��
����Ϊ���飬���ܵĽṹ��ʽ��CH3CH2CH2CH3��CH��CH3��3��
�𣺽ṹ��ʽΪCH3CH2CH2CH3��CH��CH3��3��
���㣺ͬλ�� ͬ���칹�����ͬ���칹�� ͬ�������� �й��л������ʽȷ���ļ��� �ṹ��ʽ
����������ͬ�������塢ͬϵ�ͬ���칹�����ͬ���칹��ĸ���л������ʽ��ȷ�����ѶȲ���ע���л�����ͬ������ı��������⣬ע��������ʽ�ĽǶȣ�ע��������ṹ��ʽ����д��


 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��
�� ����д���֣�
����д���֣� ��
�� ����д���֣�
����д���֣�



| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
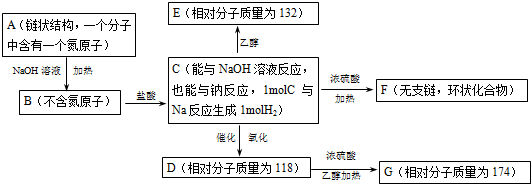

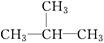
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| Ũ���� |
| �� |
| Cu |
| �� |
| Ũ���� |
| �� |
| Cu |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ֣���е�����ѧ�߶���ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
��1����A��F��
| A��11H��21H |
| B������ͼ��� |
| C�����ʯ��ʯī |
| D����������춡�� |
 ��
��
 ��
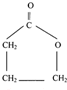
��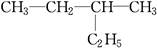
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com