
��14�֣��������ѧ�ĵ绯ѧԭ��������������⡣
��1������������ԭ��Ӧ��2Ag+(aq) + Cu(s) = Cu2+(aq) + 2Ag(s) ��Ƶ�˫Һԭ��أ������ṩ�ȶ��ĵ�����װ����ͼ��ʾ������������װ����֬������KCl��Һ��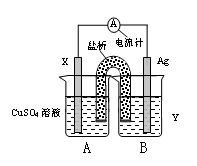
�ش��������⣺
�ٵ缫X�IJ��Ϻ͵������ҺY�ֱ�Ϊ ��
��������K+���� ����A��B����
�����缫�����ĵ缫��ӦΪ ��
��2����ͼ��ʾһ�����أ�װ�е��Һa ��X��Y������缫�壬ͨ��������ֱ����Դ��������ش��������⣺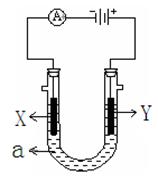
����X��Y���Ƕ��Ե缫��a�DZ���NaCl��Һ��ʵ�鿪ʼʱ��ͬʱ�����߸����뼸�η�̪��Һ�� ����X��Y���缫�����ȱ��ɫ�� X���ϵĵ缫��ӦʽΪ ��
����Ҫ������Ʒ�϶�һ����ȵ�Cu�㣬Y�缫��ӦʽΪ
����X��Y���Dz��缫�����ij����M���Ȼ��MCl2����Һ�����ռ���1.12 L����ʱ����״��������������3.2 g���ý��������ԭ�������� ��
 ��������������������ϵ�д�
��������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013�����ʡ�����и߶���һѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
��14�֣��������ѧ�ĵ绯ѧԭ��������������⡣
��1������������ԭ��Ӧ��2Ag+(aq) + Cu(s) = Cu2+(aq) + 2Ag(s) ��Ƶ�˫Һԭ��أ������ṩ�ȶ��ĵ�����װ����ͼ��ʾ������������װ����֬������KCl��Һ��
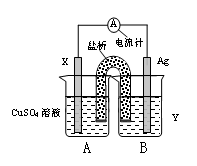
�ش��������⣺
�� �缫X�IJ��Ϻ͵������ҺY�ֱ�Ϊ ��
�� ������K+���� ����A��B����
�� ���缫�����ĵ缫��ӦΪ ��
��2����ͼ��ʾһ�����أ�װ�е��Һa ��X��Y������缫�壬ͨ��������ֱ����Դ��������ش��������⣺

����X��Y���Ƕ��Ե缫��a�DZ���NaCl��Һ��ʵ�鿪ʼʱ��ͬʱ�����߸����뼸�η�̪��Һ�� ����X��Y���缫�����ȱ��ɫ�� X���ϵĵ缫��ӦʽΪ ��
�� ��Ҫ������Ʒ�϶�һ����ȵ�Cu�㣬Y�缫��ӦʽΪ
�� ��X��Y���Dz��缫�����ij����M���Ȼ��MCl2����Һ�����ռ���1.12 L����ʱ����״��������������3.2 g���ý��������ԭ�������� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com