
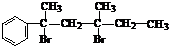
���ǻ�����ȩ��һ�ֺϳ�ҽҩ�����ϡ�Һ�����ϵ���Ҫ�м��壬�ԶԼ����ӣ��������������������������� ![]() ���������������� �� Ϊ��Ҫԭ�Ϻϳɶ��ǻ�����ȩ
���������������� �� Ϊ��Ҫԭ�Ϻϳɶ��ǻ�����ȩ
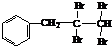
�������������������������������� ![]() ���������������� �� �Ĺ��� ������ͼ��ʾ��
���������������� �� �Ĺ��� ������ͼ��ʾ��
 |
��1��д����Ӧ��ѧ����ʽ____________________________________________,
B�ܸ�������Һ��Ӧ��������д���÷�Ӧ�Ļ�ѧ����ʽ____________________
_________________________________________________
(2)�������в�ֱ�������������Լ����ӵ�ԭ����_______________________________
____________________________________________
(3)д�����ǻ�����ȩ��һ��������������������Ӧ�Ļ�ѧ����ʽ
___________________________________________________________
(4)���ǻ�����ȩ�ж���ͬ���칹�壬���б�����ֻ��һ��������ͬ���칹��Ľṹ��ʽΪ______________________________________________.

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
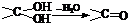 ��
�� ��NaOH��ˮ��Һ���ȵĻ�ѧ����ʽΪ��
��NaOH��ˮ��Һ���ȵĻ�ѧ����ʽΪ��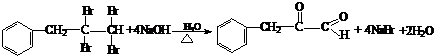
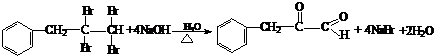
 ��NaOH���Ҵ���Һ���ȣ���������
��NaOH���Ҵ���Һ���ȣ���������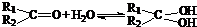 ������һ�����淴Ӧ��ƽ��״̬��ƽ���λ�ã�������ȩͪ�Ľṹ����K(H2O)=
������һ�����淴Ӧ��ƽ��״̬��ƽ���λ�ã�������ȩͪ�Ľṹ����K(H2O)=| c[R1R2C(OH)2] |
| c[R1R2C=O] |
| ������ |
|
|
|
| ||||||||
| K��H2O�� | 2��103 | 1.3 | 0.71 | 8.3��10-3 | ||||||||
| ������ |
|
|
|
| ||||||||
| K��H2O�� | 2��10-3 | 2.9 | 10 | �ܴ� |
H C6H5 |
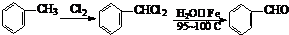 ����
����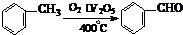
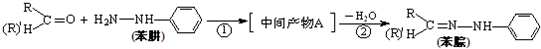
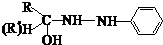
 ��
��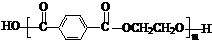
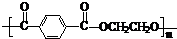 ��
��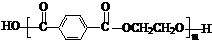
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��12�֣��������A��B��С�⣬�ֱ��Ӧ�ڡ����ʽṹ�����ʡ��͡�ʵ�黯ѧ������ѡ��ģ������ݡ���ѡ������һ�⣬������Ӧ�Ĵ������������������ⶼ������A�����֡�
A�����ںϳɰ��Ĺ�ҵú���к���H2S��C2H5SH�����ᴼ����COS���ʻ���CS2�Ⱥ������ҵ������������п���������л������������������
H2S+ZnO=ZnS+H2O��C2H5SH+ZnO=ZnS+C2H4+H2O
C2H5SH+H2=C2H6+H2S��COS+H2=CO+H2S;CS2+4H2=CH4+2H2S
��1����ԭ���ڻ�̬ʱ��������Ų�ʽΪ ��
��2�������йط��ӽṹ��˵����ȷ���� ��
A��C2H4��������5��![]() ����1��
����1��![]() ��
��
B��COS���ӣ��ṹ����ͼ���м���C=O>C=S
C��H2S���ӳ�V�νṹ
D��CH4��C2H6������̼ԭ�Ӿ�����sp3�ӻ�
��3�������й�˵������ȷ���� ��
A��H2O��CO��COS���Ǽ��Է���
B����ͬѹǿ�·е㣺Cs2>COS>CO2
C����ͬѹǿ�·е㣺C2H5SH>C2H5OH
D����ͬѹǿ�·е㣺CO>N2
��4��![]() -ZnS�ľ����ṹ����ͼ��������S2-��ĿΪ�� ����
-ZnS�ľ����ṹ����ͼ��������S2-��ĿΪ�� ����
��5���������ƾ����ṹ��ZnS��ZnO��ZnS�۵�Ϊ1830�棬ZnO�۵�Ϊ1975�棬���߽�ǰ�߸������� ��
��6�����һ������ﻯѧʽΪ��Na3[Mo(CN)8]��8H2O������ԭ�ӵ���λ��Ϊ ��
B����ȩ��Ϸ�Ӧ�л��ϳ�����Ϊ��Ҫ����ɫ�����Ĺ��������ᱶ���о��߹�ע��������нϸߵĴ����Լ��ȶ��ԡ���Ӧԭ�����£�
ʵ�鷽������25mL��ƿ�м������ᡢ10mL�״��� 0.5mL����ȩ���ڻ���״̬�·�Ӧ2h����Ӧ�IJ��ʺ�ת���ʾ��dz��ߡ�
��1�����û�����Ӧ2h��Ŀ���� ��
��2���ڷ�Ӧ�м״����������ԭ���� ��
��3����ͬ���������Բ��ʺ�ת����Ӱ�죬���±���
| ��������/mol | 0.01 | 0.02 | 0.03 | 0.05 | 0.1 | 0.15 | 0.2 | 0.6 |
| ����% | 87.3 | 88.2 | 90.3 | 94.2 | 92.9 | 93.1 | 91.8 | 92.3 |
| ת����% | 89.7 | 92.1 | 93.9 | 98.9 | 94.9 | 95.7 | 93.9 | 94.3 |
����������ȩ��״����Ϸ�Ӧʵ���д���������������Ϊ ��
��4�������Ļ������������ǿ��������һ�Ϊ��Ҫ��ָ�ꡣ�������ѭ��ʹ�ô����Բ��ʵ�Ӱ��������ͼ����˵������������ŵ�֮һ�� ��
��5��������������ʱ����ͬ��ȩ��״������Ϸ�Ӧ��ת���ʺͲ������±���
| ��� | ȩ | �� | ת����% | ����% |
| 1 | ���ǻ�����ȩ | �״� | 94.3 | 89.6 |
| 2 | ���ǻ�����ȩ | �״� | 93.6 | 88.7 |
| 3 | ���ȱ���ȩ | �״� | 93.1 | 87.3 |
| 4 | ����������ȩ | �״� | 54.2 | 34.1 |
| 5 | ����������ȩ | �״� | 89.9 | 79.5 |
| 6 | ����������ȩ | �״� | 65.7 | 41.9 |
�ӱ��еó��IJ�ͬ��ȩ��״����Ϸ�ӦӰ��ת���ʺͲ��ʵĹ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ�γ��и�����ѧ�������Ի�ѧ���� ���ͣ������
��12�֣��������A��B��С�⣬�ֱ��Ӧ�ڡ����ʽṹ�����ʡ��͡�ʵ�黯ѧ������ѡ��ģ������ݡ���ѡ������һ�⣬������Ӧ�Ĵ������������������ⶼ������A�����֡�
A�����ںϳɰ��Ĺ�ҵú���к���H2S��C2H5SH�����ᴼ����COS���ʻ���CS2�Ⱥ������ҵ������������п���������л������������������
H2S+ZnO=ZnS+H2O��C2H5SH+ZnO=ZnS+C2H4+H2O
C2H5SH+H2=C2H6+H2S��COS+H2=CO+H2S;CS2+4H2=CH4+2H2S
��1����ԭ���ڻ�̬ʱ��������Ų�ʽΪ ��
��2�������йط��ӽṹ��˵����ȷ���� ��
A��C2H4��������5�� ����1��
����1�� ��
��
B��COS���ӣ��ṹ����ͼ���м���C=O>C=S
C��H2S���ӳ�V�νṹ
D��CH4��C2H6������̼ԭ�Ӿ�����sp3�ӻ�
��3�������й�˵������ȷ���� ��
A��H2O��CO��COS���Ǽ��Է���
B����ͬѹǿ�·е㣺Cs2>COS >CO2
>CO2
C����ͬѹǿ�·е㣺C2H 5SH>C2H5OH
5SH>C2H5OH
D����ͬѹǿ�·е㣺CO>N2
��4�� -ZnS�ľ����ṹ����ͼ��������S2-��ĿΪ�� ����
-ZnS�ľ����ṹ����ͼ��������S2-��ĿΪ�� ����
��5���������ƾ����ṹ��ZnS��ZnO��ZnS�۵�Ϊ1830�棬ZnO�۵�Ϊ1975�棬���߽�ǰ�߸������� ��
��6�����һ������ﻯѧʽΪ��Na3[Mo(CN)8]��8H2O������ԭ�ӵ���λ��Ϊ ��
B����ȩ��Ϸ�Ӧ�л��ϳ�����Ϊ��Ҫ����ɫ�����Ĺ��������ᱶ���о��߹�ע��������нϸߵĴ����Լ��ȶ��ԡ���Ӧԭ�����£�
ʵ�鷽������25mL��ƿ�м������ᡢ10mL�״��� 0.5mL����ȩ���ڻ���״̬�·�Ӧ2h����Ӧ�IJ��ʺ�ת���ʾ��dz��ߡ�
��1�����û�����Ӧ2h��Ŀ���� ��
��2���ڷ�Ӧ�м״����������ԭ���� �� ��3����ͬ���������Բ��ʺ�ת����Ӱ�죬���±���
��3����ͬ���������Բ��ʺ�ת����Ӱ�죬���±���
| ��������/mol | 0.01 | 0.02 | 0.03 | 0.05 | 0.1 | 0.15 | 0.2 | 0.6 |
| ����% | 87.3 | 88.2 | 90.3 | 94.2 | 92.9 | 93.1 | 91.8 | 92.3 |
| ת����% | 89.7 | 92.1 | 93.9 | 98.9 | 94.9 | 95.7 | 93.9 | 94.3 |
| ��� | ȩ | �� | ת����% | ����% |
| 1 | ���ǻ�����ȩ | �״� | 94.3 | 89.6 |
| 2 | ���ǻ�����ȩ | �״� | 93.6 | 88.7 |
| 3 | ���ȱ���ȩ | �״� | 93.1 | 87.3 |
| 4 | ����������ȩ | �״� | 54.2 | 34.1 |
| 5 | ����������ȩ | �״� | 89.9 | 79.5 |
| 6 | ����������ȩ | �״� | 65.7 | 41.9 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ�γ��и�����ѧ�������Ի�ѧ���� ���ͣ������
��12�֣��������A��B��С�⣬�ֱ��Ӧ�ڡ����ʽṹ�����ʡ��͡�ʵ�黯ѧ������ѡ��ģ������ݡ���ѡ������һ�⣬������Ӧ�Ĵ������������������ⶼ������A�����֡�
A�����ںϳɰ��Ĺ�ҵú���к���H2S��C2H5SH�����ᴼ����COS���ʻ���CS2�Ⱥ������ҵ������������п���������л������������������
H2S+ZnO=ZnS+H2O��C2H5SH+ZnO=ZnS+C2H4+H2O
C2H5SH+H2=C2H6+H2S��COS+H2=CO+H2S;CS2+4H2=CH4+2H2S
��1����ԭ���ڻ�̬ʱ��������Ų�ʽΪ ��
��2�������йط��ӽṹ��˵����ȷ���� ��
A��C2H4��������5�� ����1��
����1�� ��
��
B��COS���ӣ��ṹ����ͼ���м���C=O>C=S
C��H2S���ӳ�V�νṹ
D��CH4��C2H6������̼ԭ�Ӿ�����sp3�ӻ�
��3�������й�˵������ȷ���� ��
A��H2O��CO��COS���Ǽ��Է���
B����ͬѹǿ�·е㣺Cs2>COS>CO2
C����ͬѹǿ�·е㣺C2H5SH>C2H5OH
D����ͬѹǿ�·е㣺CO>N2
��4��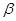 -ZnS�ľ����ṹ����ͼ��������S2-��ĿΪ�� ����
-ZnS�ľ����ṹ����ͼ��������S2-��ĿΪ�� ����

��5���������ƾ����ṹ��ZnS��ZnO��ZnS�۵�Ϊ1830�棬ZnO�۵�Ϊ1975�棬���߽�ǰ�߸������� ��
��6�����һ������ﻯѧʽΪ��Na3[Mo(CN)8]��8H2O������ԭ�ӵ���λ��Ϊ ��
B����ȩ��Ϸ�Ӧ�л��ϳ�����Ϊ��Ҫ����ɫ�����Ĺ��������ᱶ���о��߹�ע��������нϸߵĴ����Լ��ȶ��ԡ���Ӧԭ�����£�

ʵ�鷽������25mL��ƿ�м������ᡢ10mL�״��� 0.5mL����ȩ���ڻ���״̬�·�Ӧ2h����Ӧ�IJ��ʺ�ת���ʾ��dz��ߡ�
��1�����û�����Ӧ2h��Ŀ���� ��
��2���ڷ�Ӧ�м״����������ԭ���� ��
��3����ͬ���������Բ��ʺ�ת����Ӱ�죬���±���
|
��������/mol |
0.01 |
0.02 |
0.03 |
0.05 |
0.1 |
0.15 |
0.2 |
0.6 |
|
����% |
87.3 |
88.2 |
90.3 |
94.2 |
92.9 |
93.1 |
91.8 |
92.3 |
|
ת����% |
89.7 |
92.1 |
93.9 |
98.9[��Դ:Zxxk.Com] |
94.9 |
95.7 |
93.9 |
94.3 |
����������ȩ��״����Ϸ�Ӧʵ���д���������������Ϊ ��
��4�������Ļ������������ǿ��������һ�Ϊ��Ҫ��ָ�ꡣ�������ѭ��ʹ�ô����Բ��ʵ�Ӱ��������ͼ����˵������������ŵ�֮һ�� ��
��5��������������ʱ����ͬ��ȩ��״������Ϸ�Ӧ��ת���ʺͲ������±���
|
��� |
ȩ |
�� |
ת����% |
����% |
|
1 |
���ǻ�����ȩ |
�״� |
94.3 |
89.6 |
|
2 |
���ǻ�����ȩ |
�״� |
93.6 |
88.7 |
|
3 |
���ȱ���ȩ |
�״� |
93.1 |
87.3 |
|
4 |
����������ȩ |
�״� |
54.2 |
34.1 |
|
5 |
����������ȩ |
�״� |
89.9 |
79.5 |
|
6 |
����������ȩ |
�״� |
65.7 |
41.9 |

�ӱ��еó��IJ�ͬ��ȩ��״����Ϸ�ӦӰ��ת���ʺͲ��ʵĹ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�������A��B��С�⣬�ֱ��Ӧ�ڡ����ʽṹ�����ʡ��͡�ʵ�黯ѧ������ѡ��ģ������ݡ���ѡ������һ�⣬������Ӧ�Ĵ������������������ⶼ������A�����֡�
A�����ںϳɰ��Ĺ�ҵú���к���H2S��C2H5SH�����ᴼ����COS���ʻ���CS2�Ⱥ������ҵ������������п���������л������������������
H2S+ZnO=ZnS+H2O��C2H5SH+ZnO=ZnS+C2H4+H2O
C2H5SH+H2=C2H6+H2S��COS+H2=CO+H2S;CS2+4H2=CH4+2H2S
��1����ԭ���ڻ�̬ʱ��������Ų�ʽΪ ��
��2�������йط��ӽṹ��˵����ȷ���� ��
A��C2H4��������5��![]() ����1��
����1��![]() ��
��
B��COS���ӣ��ṹ����ͼ���м���C=O>C=S
C��H2S���ӳ�V�νṹ
D��CH4��C2H6������̼ԭ�Ӿ�����sp3�ӻ�
��3�������й�˵������ȷ���� ��
A��H2O��CO��COS���Ǽ��Է���
B����ͬѹǿ�·е㣺Cs2>COS>CO2
C����ͬѹǿ�·е㣺C2H5SH>C2H5OH
D����ͬѹǿ�·е㣺CO>N2
��4��![]() -ZnS�ľ����ṹ����ͼ��������S2-��ĿΪ�� ����
-ZnS�ľ����ṹ����ͼ��������S2-��ĿΪ�� ����

��5���������ƾ����ṹ��ZnS��ZnO��ZnS�۵�Ϊ1830�棬ZnO�۵�Ϊ1975�棬���߽�ǰ�߸������� ��
��6�����һ������ﻯѧʽΪ��Na3[Mo(CN)8]��8H2O������ԭ�ӵ���λ��Ϊ ��
B����ȩ��Ϸ�Ӧ�л��ϳ�����Ϊ��Ҫ����ɫ�����Ĺ��������ᱶ���о��߹�ע��������нϸߵĴ����Լ��ȶ��ԡ���Ӧԭ�����£�

ʵ�鷽������25mL��ƿ�м������ᡢ10mL�״��� 0.5mL����ȩ���ڻ���״̬�·�Ӧ2h����Ӧ�IJ��ʺ�ת���ʾ��dz��ߡ�
��1�����û�����Ӧ2h��Ŀ���� ��
��2���ڷ�Ӧ�м״����������ԭ���� ��
��3����ͬ���������Բ��ʺ�ת����Ӱ�죬���±���
| ��������/mol | 0.01 | 0.02 | 0.03 | 0.05 | 0.1 | 0.15 | 0.2 | 0.6 |
| ����% | 87.3 | 88.2 | 90.3 | 94.2 | 92.9 | 93.1 | 91.8 | 92.3 |
| ת����% | 89.7 | 92.1 | 93.9 | 98.9 | 94.9 | 95.7 | 93.9 | 94.3 |
����������ȩ��״����Ϸ�Ӧʵ���д���������������Ϊ ��
��4�������Ļ������������ǿ��������һ�Ϊ��Ҫ��ָ�ꡣ�������ѭ��ʹ�ô����Բ��ʵ�Ӱ��������ͼ����˵������������ŵ�֮һ�� ��
��5��������������ʱ����ͬ��ȩ��״������Ϸ�Ӧ��ת���ʺͲ������±���
| ��� | ȩ | �� | ת����% | ����% |
| 1 | ���ǻ�����ȩ | �״� | 94.3 | 89.6 |
| 2 | ���ǻ�����ȩ | �״� | 93.6 | 88.7 |
| 3 | ���ȱ���ȩ | �״� | 93.1 | 87.3 |
| 4 | ����������ȩ | �״� | 54.2 | 34.1 |
| 5 | ����������ȩ | �״� | 89.9 | 79.5 |
| 6 | ����������ȩ | �״� | 65.7 | 41.9 |

�ӱ��еó��IJ�ͬ��ȩ��״����Ϸ�ӦӰ��ת���ʺͲ��ʵĹ����� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com