A������ѧ�����
��1���Ͼ�����»ᡱ�ѽ��뵹��ʱ���������ݺͳ��н�ͨ�����������ƣ�
���������ݽ���������������ϣ����в��ϲ����ڹ����β��ϵ���
������ĸ����
a��ʯ��ʯ b��ˮ�� c������
�ڹ����ͨ����������������ϣ����н���������������ʴ����
������ĸ����
a�����Ͻ� b������ c����ͭ
�ۡ��ܽ����ܵ����ɾ۰����Ȳ��Ͻ��ɣ��۰���������
������ĸ����
a���������� b�����ǽ������� c���л��߷��Ӳ���
��2������»ᡱ�ڼ䣬Ҫ�����˶�Ա��Ӫ���뽡����
�ټ�ʱ�����������˶�Աȡ������ɼ��Ļ�����֤���������Ӫ�����������������������ܵ������ࡢ
��
��
��ˮ�����߲˸���V
C����֪V
C�ĽṹΪ
�������ʽΪ
�����Ȼ�����Һ�м���V
C��Һ����Һ�ɻ�ɫת��Ϊdz��ɫ��˵��V
C���н�ǿ��
�ԣ�
�۷���Υ��ҩ�ﲻ���������������Ĺ�ƽ��������Ҳ�к��˶�Ա�����Ľ������ڰ�˹ƥ�֡���ù�ء�����ء�С�մ�ȳ���ҩ���У�����ѡ�ֲ��ɷ��õ���
��
��3������ˮ���족����Ϊ�˶�Ա�ṩ����������������չʾ�Ŷ��Ͼ�����������
��PM2.5ָ�����ڴ����е�ֱ����2.5��m���ף��Ŀ��������PM2.5����ɻ���������Σ�����彡����ȼú���������ڿ���PM2.5�ĺ�����д����̿��ˮ������Ӧ�Ļ�ѧ����ʽ
��
������β���к�����Ⱦ������NO��CO�������������ܼ�װ����ת����������ʹCO��NO��Ӧ����������Ⱦ�����壬��Ӧ�Ļ�ѧ����ʽΪ
��װ�С���ת����������������ʹ����Ǧ���ͣ���ԭ����
��
�ۺ���Ԫ�صķ�ˮ����������꣮ij��ȤС��̽��������Cr
2O
72������ˮ�Ĵ������������������ϣ�����ã�NH
4��
2Fe��SO
4��
2��Cr
2O
72����ԭΪCr
3+�����ð�ˮ��Cr
3+ת������ܵ�Cr��OH��
3���÷�����������ˮ���������е�������ԭ����
��ָ����ʦָ���÷���������ķ�ˮ�����д���
�������ӷ��ţ����ܵ���ˮ�帻Ӫ������

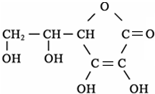
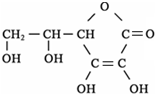 ��1���Ͼ�����»ᡱ�ѽ��뵹��ʱ���������ݺͳ��н�ͨ�����������ƣ�
��1���Ͼ�����»ᡱ�ѽ��뵹��ʱ���������ݺͳ��н�ͨ�����������ƣ�

 �߽�������ϵ�д�
�߽�������ϵ�д�
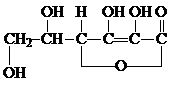 �������ʽΪ
�������ʽΪ �������ʽΪ
�������ʽΪ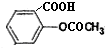 ���dz��õ�
���dz��õ� �������ʽΪ
�������ʽΪ