
���� ��1����������һ�����ʵ���Ũ�ȵ���Һʹ�õ��������з�����
��2��������Һϡ������������������ʵ�������������ҪŨ����������
��3����������һ�����ʵ�����Ũ����Һ�ķ�����������
��4������c=$\frac{n}{V}$�������������ʵ����ʵ��������Һ�������Ӱ���жϣ�
��� �⣺��1����18.4mol/L��Ũ�������Ƴ�Ũ��Ϊ0.5mol•L-1��ϡ����250ml��ʹ�õ���������Ͳ���ձ�����������250mL����ƿ����ͷ�ιܣ����Ի���Ҫ������Ϊ��250mL����ƿ����ͷ�ιܡ��ձ���
�ʴ�Ϊ��250mL����ƿ����ͷ�ιܣ��ձ���
��2������ҪŨ��������ΪV����������Һϡ������������������ʵ�������ã�18.4mol/L��V=0.5mol•L-1��250mL�����V=6.8mL��
�ʴ�Ϊ��6.8��
��3�����Ʋ���Ϊ����ȡŨ���ᡢŨ�����ϡ�͡�ת�ơ����ݡ�ҡ�ȡ�ת���Լ�ƿ��������ȷ˳��Ϊ��AEDCB��
�ʴ�Ϊ��AEDCB��
��4����Ũ����ϡ�ͺ�ֱ��ת������ƿ�����ݣ�Ũ����ϡ��ʱ�����������ȣ���ȴ��Һ���½���������Һ���ƫС����ʹŨ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
�ڶ���ҡ�Ⱥ���Һ����ڿ̶��ߣ�������ˮ���̶��ߵ�����Һ���ƫ��ʹ��ҺŨ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
������Ͳ��ȡŨ������������Ͳ������ȡ��Ũ�������ƫС����������ʵ���ƫС����ʹ��ҺŨ��ƫ�ͣ���Һ����ʱ��������ƿ������Һ���ƫ����ʹŨ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�ƫ�ͣ�
���� ���⿼��һ�����ʵ���Ũ����Һ���ƹ��̡����ʵ���Ũ���йؼ�����������ȣ���ȷ����ԭ�������������ǽ���ؼ����ѶȲ���������Ϊ�״��㣮



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 8 | B�� | 13 | C�� | 14 | D�� | 18 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
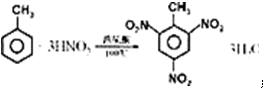 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
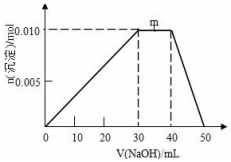 ��ʢ��10mL1mol•L-1 NH4Al��SO4��2��Һ���ձ��еμ�1mol•L-1NaOH��Һ���������ʵ�����NaOH��Һ����仯ʾ��ͼ���£�
��ʢ��10mL1mol•L-1 NH4Al��SO4��2��Һ���ձ��еμ�1mol•L-1NaOH��Һ���������ʵ�����NaOH��Һ����仯ʾ��ͼ���£��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڿ��������ձ���CO2�������ڿ�����Ⱦָ�� | |
| B�� | �ճ������к�ҽԺ������ˮ�Ҵ�ɱ������ | |
| C�� | ��ɫʳƷ��ָ�����κλ�ѧ���ʵ�ʳƷ | |
| D�� | Ŀǰ�ӵ�ʳ������Ҫ���ӵ���KIO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
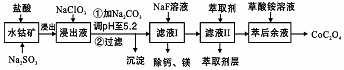
| ������ | Fe��OH��3 | Fe��OH��2 | Co��OH��2 | Al��OH��3 | Mn��OH��2 |
| ��ȫ������pH | 3.7 | 9.6 | 9.2 | 5.2 | 9.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
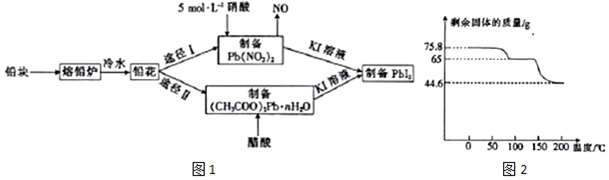
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com