
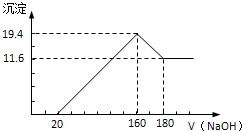
 ������������250mL 0.1mol/L��Na2CO3��Һ����ղ���ش��������⣺
������������250mL 0.1mol/L��Na2CO3��Һ����ղ���ش��������⣺| ʵ��Ӧ��Na2CO3����/g | Ӧѡ������ƿ�Ĺ��/mL | ������ƿ���Ҫ���������� |
| 2.7g | 250ml | ���������ձ�����ͷ�ι� |
| n |
| V |
| n |
| V |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �������������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�������ͼΪ����ʵ��װ�ã�
�������������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�������ͼΪ����ʵ��װ�ã��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
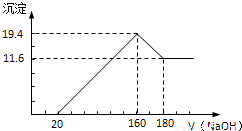
 ������������250mL 0.1mol/L��Na2CO3��Һ����ղ���ش��������⣺
������������250mL 0.1mol/L��Na2CO3��Һ����ղ���ش��������⣺| ʵ��Ӧ��Na2CO3����/g | Ӧѡ������ƿ�Ĺ��/mL | ������ƿ���Ҫ���������� |
| 2.7g | 250ml | ���������ձ�����ͷ�ι� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ������ɽһ�и�һ���ϣ���ĩ��ѧ�Ծ��������棩 ���ͣ������
| ʵ��Ӧ��Na2CO3����/g | Ӧѡ������ƿ�Ĺ��/mL | ������ƿ���Ҫ���������� |
| 2.7g | 250ml | ���������ձ�����ͷ�ι� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ������������250mL0.1mol/L��Na2CO3��Һ,��ղ���ش��������⣺
![]() ��1����ѡ�õ�����ƿ����� ����250mL��500mL��100mL��1000mL��
��1����ѡ�õ�����ƿ����� ����250mL��500mL��100mL��1000mL��
![]() ��2������ʱ������ȷ�IJ���˳���ǣ���ĸ��ʾ��ÿ����ĸֻ����һ�Σ� ___ ___ __��
��2������ʱ������ȷ�IJ���˳���ǣ���ĸ��ʾ��ÿ����ĸֻ����һ�Σ� ___ ___ __��
![]() A��������ˮϴ���ձ��Ͳ�����2��3�Σ���ϴ��Һת������ƿ����ҡ��
A��������ˮϴ���ձ��Ͳ�����2��3�Σ���ϴ��Һת������ƿ����ҡ��
![]() B������ƽ�ϳ�ȡһ������Na2CO3�����ձ�����ˮ�ܽ⣬��ȴ������
B������ƽ�ϳ�ȡһ������Na2CO3�����ձ�����ˮ�ܽ⣬��ȴ������
![]() C�����Ƶõ���ҺС�ĵ�ע������ƿ��
C�����Ƶõ���ҺС�ĵ�ע������ƿ��
![]() D��������ƿ�����������ҡ��
D��������ƿ�����������ҡ��
![]() E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�����
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�����
![]() F������������ƿ�м�ˮ����̶���1cm��2cm��
F������������ƿ�м�ˮ����̶���1cm��2cm��
![]() ��3�������������������������ҺŨ�Ƚ��к�Ӱ�죨�ƫ�ߡ�����ƫ�͡�����Ӱ�족����û�н���A���� ����������ˮʱ���������� �̶� ��������ʱ���ӿ̶���___________________��
��3�������������������������ҺŨ�Ƚ��к�Ӱ�죨�ƫ�ߡ�����ƫ�͡�����Ӱ�족����û�н���A���� ����������ˮʱ���������� �̶� ��������ʱ���ӿ̶���___________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com