
(1)��ȡa g��Ʒ�������м���������NaOH��Һ��������ɵ�����(��״������ͬ)���Ϊb L����Ӧ�Ļ�ѧ����ʽ��____________________________________����Ʒ������������___________ g��
(2)��ȡa g��Ʒ�����ȼ��ǡ����ȫ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽ��____________________����������������������___________��
(3)��(2)�з�Ӧ������ȴ�����������ᣬ������ɵ��������Ϊc L����������(1)����������������c��b=___________��
(1)2Al��2NaOH��2H2O![]() 2NaAlO2��3H2��
2NaAlO2��3H2�� ![]()
(2)2Al��Fe2O3![]() Al2O3��2Fe 80��27
Al2O3��2Fe 80��27
(3)2��3
������(1)
2Al��2NaOH��2H2O![]() 2NaAlO2��3H2��
2NaAlO2��3H2��
m(Al) ![]()
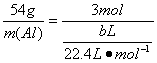
m(Al)=![]() g
g
(2)2Al + Fe2O3![]() Al2O3+2Fe
Al2O3+2Fe
m(Fe2O3)��m(Al)=
(3)��Al�����ʵ���Ϊ2 mol����ô����Fe�����ʵ���Ϊ2 mol��
2Al �� 3H2�� Fe �� H2��
2 mol 3 mol 2 mol 2 mol
![]() =2��3
=2��3



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)��ȡa g��Ʒ�������м���������NaOH��Һ���������(��״������ͬ)��������ΪbL���÷�Ӧ�Ļ�ѧ����ʽ��________________________________________������Ʒ������������__________g��
(2)��ȡa g��Ʒ������ȼ��ʹ��ǡ����ȫ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽ��__________________����Ӧ����������������������__________��
(3)��(2)�з�Ӧ������ȴ�����������ϡ�����У���Ӧ��ȫ������ɵ��������Ϊc L�����������(1)����������������c��b=__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)ȡa g��Ʒ�������м���������NaOH��Һ��������ɵ�����(��״������ͬ)���Ϊb L����Ӧ�Ļ�ѧ����ʽ��______________________����Ʒ������������__________g��
(2)ȡa g��Ʒ�ڸ��������£�ǡ����ȫ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ____________________����������������������_________��
(3)��(2)�з�Ӧ������ȴ�����������ᣬ������ɵ��������Ϊc L����������(1)����������������c��b=_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��0109 ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊȷ��ij���ȼ�(������������)����ɣ��ֱ��������ʵ�顣
��1����ȡa g��Ʒ�������м���������NaOH��Һ��������ɵ�����(��״������ͬ)���Ϊb L�� ��Ӧ�Ļ�ѧ����ʽ��___________________����Ʒ������������_____________g��
��2����ȡa g��Ʒ�����ȼ��ǡ����ȫ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽ��_ ��������������������_____________��
��3����(2)�з�Ӧ������ȴ�����������ᣬ������ɵ��������Ϊc L����������(1)����������������c��b=_____________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com