
����14�֣���ͼ����ѧ��ѧ�г�������֮���һЩ��Ӧ��ϵ�����в��ֲ���δд����������X�ǹ��壬X���ȷֽ����������ڱ���µ������Ϊ1��1��HΪ����ɫ��ĩ��B��G��������Һ�壬�����Ϊ���塣����ͼ�й�ϵ�ƶϣ�
��1����ѧʽX ��
��2��A�ĵ���ʽΪ ���ռ乹�� ��
C�ĽṹʽΪ ��H�������Ļ�ѧ������ ��
��3����ҵ����ȡA�Ļ�ѧ��Ӧ����ʽ ��A��D�Ļ�ѧ��Ӧ����ʽ ��
C+H��E�Ļ�ѧ��Ӧ����ʽ ��G��D�����ӷ�Ӧ����ʽ ��
 Сѧ��ʱ��ҵȫͨ����ϵ�д�
Сѧ��ʱ��ҵȫͨ����ϵ�д� �����ÿ�ʱѵ��ϵ�д�
�����ÿ�ʱѵ��ϵ�д� ��Ԫȫ��������ϵ�д�
��Ԫȫ��������ϵ�д� �»ƸԱ����ܾ�ϵ�д�
�»ƸԱ����ܾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2010�����ʡ����һ�и�һ��ѧ����ĩ���Ի�ѧ���� ���ͣ������
����14�֣���ͼ����ѧ��ѧ�г�������֮���һЩ��Ӧ��ϵ�����в��ֲ���δд����������X�ǹ��壬X���ȷֽ����������ڱ���µ������Ϊ1��1��HΪ����ɫ��ĩ��B��G��������Һ�壬�����Ϊ���塣����ͼ�й�ϵ�ƶϣ�
��1����ѧʽX ��
��2��A�ĵ���ʽΪ ���ռ乹�� ��
C�ĽṹʽΪ ��H�������Ļ�ѧ������ ��
��3����ҵ����ȡA�Ļ�ѧ��Ӧ����ʽ ��A��D�Ļ�ѧ��Ӧ����ʽ ��
C+H��E�Ļ�ѧ��Ӧ����ʽ ��G��D�����ӷ�Ӧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ��У����9�µ�һ��������ѧ�Ծ��������棩 ���ͣ������
��ÿ��2�֣���14�֣���ͼ�У�A��B��C��D��E�ǵ��ʣ�G��H��I��F��B��C��D��E�ֱ��A�γɵĶ�Ԫ�������֪��
�� G��һ�ֺ���ɫ��ĩ��C����������������NaOH��Һ��Ӧ
�� I��һ�ֳ������������壬����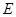 ���Է�����Ӧ��2E��I
���Է�����Ӧ��2E��I 2F��D��F�е�EԪ�ص���������Ϊ60%.
2F��D��F�е�EԪ�ص���������Ϊ60%.
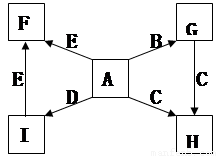
�ش����⣺
(1��д��G��H�Ļ�ѧ����ʽΪ________________________��
�˷�Ӧ�ڹ�ҵ�ϳ����ں��Ӹֹ죬ʵ��������ʾ��ʵ��ʱ����װ���м���G��C�����������˷�Ӧ�IJ����� ��
(2���������ĵ���ʽΪ______________________,
(3���������������ܽ�G��������Һ��ʴͭ����������ϴ��ӡˢ��·���ϵ�ͭ��д���÷�Ӧ�����ӷ���ʽΪ________________;
(4��E��I��ȼ�չ۲쵽��������_______________________��
(5)������ı����£���һ��������C��E���������ɻ��һ��DZ�ڵ��������E17C12 ���ò�������ķ�Ӧ����ʽΪE17C12+17H2 = 17EH2+12C
�������Ʊ��������E17C12ʱͨ�������Ŀ����
��1mol E17C12��ȫ������õ��IJ�����������������ȫ��Ӧ���ͷų�H2�����ʵ���Ϊ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ��һ��ѧ����ĩ���Ի�ѧ���� ���ͣ������
����14�֣���ͼ����ѧ��ѧ�г�������֮���һЩ��Ӧ��ϵ�����в��ֲ���δд����������X�ǹ��壬X���ȷֽ����������ڱ���µ������Ϊ1��1��HΪ����ɫ��ĩ��B��G��������Һ�壬�����Ϊ���塣����ͼ�й�ϵ�ƶϣ�
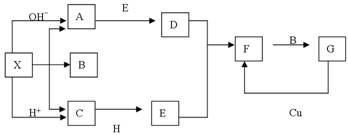
��1����ѧʽX ��
��2��A�ĵ���ʽΪ ���ռ乹�� ��
C�ĽṹʽΪ ��H�������Ļ�ѧ������ ��
��3����ҵ����ȡA�Ļ�ѧ��Ӧ����ʽ ��A��D�Ļ�ѧ��Ӧ����ʽ ��
C+H��E�Ļ�ѧ��Ӧ����ʽ ��G��D�����ӷ�Ӧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����14�֣���ͼ����ѧ��ѧ�г�������֮���һЩ��Ӧ��ϵ�����в��ֲ���δд����������X�ǹ��壬X���ȷֽ����������ڱ���µ������Ϊ1��1��HΪ����ɫ��ĩ��B��G��������Һ�壬�����Ϊ���塣����ͼ�й�ϵ�ƶϣ�
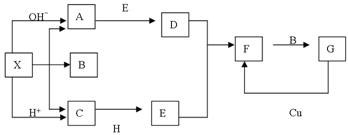
��1����ѧʽX ��
��2��A�ĵ���ʽΪ ���ռ乹�� ��
C�ĽṹʽΪ ��H�������Ļ�ѧ������ ��
��3����ҵ����ȡA�Ļ�ѧ��Ӧ����ʽ ��A��D�Ļ�ѧ��Ӧ����ʽ ��
C+H��E�Ļ�ѧ��Ӧ����ʽ ��G��D�����ӷ�Ӧ����ʽ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com