
ClO2������һ�ָ�Ч�����ס���ȫ��ɱ��������������NaClO3�Ͳ��ᣨH2C2O4����Ӧ�Ƶá���ˮ����100�����������ijѧϰС������ͼװ��ģ�ҵ��ȡ�ռ�ClO2 ��

��1��ʵ��ʱװ��A����60��~100����е�ԭ����_________�����������¶ȵķ�����___________��
��2���綯�������������_________��װ��A�з�Ӧ������Na2CO3��ClO2��CO2�ȣ��÷�Ӧ�Ļ�ѧ����ʽΪ___________________��
��3����װ��C��ClO2��NaOH��Ӧ���ɵ����ʵ����������Σ�����һ����ΪNaClO2 ��д����Ӧ�����ӷ���ʽ_________________��
��4����ClO2������������ˮ��pHΪ5.5~6.5��������������ClO2��һ���������岻��������������ӣ�ClO2������
�� ClO2��I����ԭΪClO2����Cl����ת��������ҺpH�Ĺ�ϵ����ͼ
 ��ʾ
��ʾ
�� ��pH��2.0ʱ��ClO2��Ҳ�ܱ�I����ԭ��Cl��
�� 2Na2S2O3��I2��Na2S4O6��2NaI
����������Ϣ���벹�������ⶨ����ˮ��ClO2��������ʵ�鷽����
ȡһ�����������ˮ������NaOH��Һ����pHΪ7.0~8.0�� �����ظ���������1~2�Σ�����ó������
��ʵ������ʹ�õ��Լ���������Һ����Na2S2O3��Һ��KI��Һ��ϡ���ᣩ
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017������ʡ���²�������ѧ��һ�ָ�ϰ��һ�β��Ի�ѧ�Ծ��������棩 ���ͣ������
��2.5 g̼���ơ�̼�����ƺ��������ƵĹ���������ȫ����ˮ���Ƴ�ϡ��Һ��Ȼ�������Һ����μ���1 mol��L��1�����ᣬ�������������������CO2�����(��״��)��ϵ����ͼ��ʾ��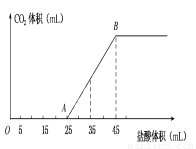
��1�� д��OA����������Ӧ�����ӷ���ʽ_______________��
��2��������35 mL����ʱ������CO2�����Ϊ___________mL(��״��)��
��3��ԭ�������NaOH������Ϊ___________g��̼���Ƶ���������Ϊ_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017������ʡ��«������УЭ����������ڳ����Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����й�˵����ȷ���ǣ� ��
A.�±���ʳƷ���ڰ�װ�з�����ʯ����������
B�������������ܹ�ͨ�����ܽ���������������ֱ����ԼΪ80nm
C����ά�ؿ�ˮ��������ǣ��ʿ�Ϊ�����ṩӪ��
D����Na2FeO4������ˮ����ɱ�������������ܳ���ˮ�е������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ������ٿ�ѧ���л�ѧ�Ծ��������棩 ���ͣ�ѡ����
��t ��ʱ����a g NH3��ȫ����ˮ���õ�V mL��Һ���������Һ���ܶ�Ϊ�� g��mL��1�����ʵ���������Ϊw�����к���NH4+�����ʵ�����b mol������������ȷ���ǣ� ��
A�����ʵ���������w�� ��100%
��100%
B�����ʵ����ʵ���Ũ��c��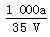 mol��L��1
mol��L��1
C����Һ��c(OH��)��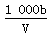 mol��L��1��c(H��)
mol��L��1��c(H��)
D����������Һ�м���V mLˮ��������Һ��������������0.5w
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ������ٿ�ѧ���л�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����йػ�ѧ�����ʾ��ȷ���ǣ� ��
A���������Ƶĵ���ʽ��
B��������Ϊ53��������Ϊ78�ĵ�ԭ�ӣ�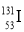
C��ˮ���ӵı���ģ�ͣ�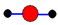
D���������ױ��Ľṹ��ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ������ѧ���ڳ��������Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
H2C2O4Ϊ��Ԫ���ᡣ20��ʱ������һ��c��H2C2O4��+ c��HC2O4�C��+ c��C2O42�C��=0.100 mol��L�C1��H2C2O4��NaOH�����Һ����Һ�в����������ʵ���Ũ����pH�ı仯������ͼ��ʾ������ָ����Һ���������ʵ���Ũ�ȹ�ϵһ����ȷ���ǣ� ��
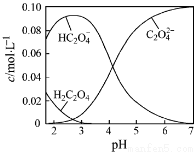
A��pH=2.5����Һ�У�c(H2C2O4)+c(C2O42�C)��c(HC2O4�C)
B��c(Na+)=0.100 mol��L�C1����Һ�У�c(H+)+c(H2C2O4)=c(OH�C)+c(C2O42�C)
C��c(HC2O4�C)=c(C2O42�C)����Һ�У�c(Na+)��0.100 mol��L�C1+c(HC2O4�C)
D��pH=7����Һ�У�c(Na+)��2c(C2O42�C)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ������ѧ���ڳ��������Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�������ʵ�ת���ڸ��������²���ʵ�ֵ��ǣ� ��
A��Ca(ClO)2(aq)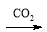 HClO(aq)
HClO(aq)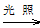 HCl(aq)
HCl(aq)
B��H2SiO3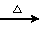 SiO2
SiO2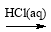 SiCl4
SiCl4
C��Al2O3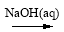 NaAlO2
NaAlO2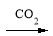 Al(OH)3
Al(OH)3
D��Fe2O3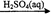 Fe2(SO4)3(aq)
Fe2(SO4)3(aq) ��ˮFe2(SO4)3
��ˮFe2(SO4)3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����и�����ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
������ѧ����������������������������װ�ã���ԭ�������Ƚ�����(NaAuCl4)��Һ�������е������Ƿ�Ӧ���������ʿ���(ֱ��Ϊ20 nm��60 nm)�������й�˵���д������( )
A�����ʱNaAuCl4����������Ӧ
B�������ǵĽṹ��ʽΪCH2OH(CHOH)4CHO
C�������Ǿ��л�ԭ��
D�����������ɢ��ˮ�����õķ�ɢϵ�ܲ��������ЧӦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�긣��ʡȪ����ѧҵˮƽ��ѧģ���Ծ����ģ��������棩 ���ͣ�ʵ����
��ͼ��һ����ȡ����֤�������ֻ�ѧ���ʵ�ʵ��װ�ã�
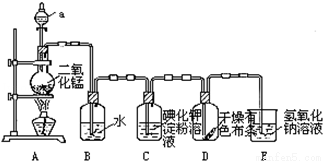
�ش��������⣺��Ӧ�ķ���ʽ��MnO2+4HCl��Ũ�� MnCl2+2H2O+Cl2��
MnCl2+2H2O+Cl2��
��1��װ��A�У�����a�����ƽ� ����������ʢ�е��Լ�Ϊ ��
��2����Cl2����ͨ��һ��ʱ���װ��B����Һ��pHֵ 7���������������=������д��Cl2��ˮ��Ӧ�ķ���ʽ
��3����������Cl2����ͨ���۲쵽װ��C�е���Һ��Ϊ ɫ����Ӧ�����ӷ���ʽΪ ��
��4����Cl2��������ͨ��ʱ��װ��D�и������ɫ�����ܷ���ɫ��Ϊʲô�� ��
��5��װ��E�������� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com