
ij��ȤС�����������ʵ��װ�á�ʵ��ʱ���ȶϿ�K2���պ�K1�������������ݲ�����һ��ʱ��Ͽ�K1���պ�K2�����ֵ�����ָ��ƫת�������й�������ȷ����
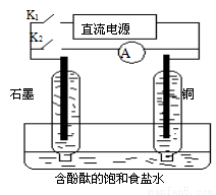
A���Ͽ�K2���պ�K1ʱ���ܷ�Ӧ�����ӷ���ʽΪ��2H++2Cl�� Cl2��+H2��
Cl2��+H2��
B���Ͽ�K2���պ�K1ʱ��ʯī�缫������Һ���
C���Ͽ�K1���պ�K2ʱ��ͭ�缫�ϵĵ缫��ӦΪ��Cl2+2e����2Cl��
D���Ͽ�K1���պ�K2ʱ��ʯī�缫������

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�߶��Ͽ�ѧ�⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
����ÿ�������к��еĻ�ѧ��������ͬ����
A. NaCl��HCl��H2O��NaOH B. Cl2��Na2S��HCl��SO2
C. HBr��CO2��H2O��CS2 D. Na2O2��H2O2��H2O��O3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017������һ�и�����ѧ�ڿ�ѧ�����ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
NAΪ����٤������������������ȷ����
A��7.8 g Na2O2��������������0.2NA
B��0.1 mol 16OD�� ���Ӻ��еĵ��ӡ���������Ϊ1.0NA
C�����³�ѹ�£�42g��ϩ�Ͷ�ϩ��������У����Լ���Ϊ 6 NA
D���ܱ�������2 mol NO��1 mol O2��ַ�Ӧ������ķ�����Ϊ2NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��ɽ��ʡ����У������ѧ�ڵ�һ��������ѧ�Ծ��������棩 ���ͣ�ѡ����
���й������ӹ�������ӷ���ʽ��˵����ȷ����
A��ij������Һ�п��ܴ������ڣ�NH4+��Fe3+��NO3-��I-
B����ˮ�����c(H+)==10-12 mol��L-1����Һ�У�Al3+��Cl-��Ba2+��NO3-һ�����ܴ�������
C����NH3��H2O��Һ�еμ�����AlCl3��Һ, ������Ӧ��Al3++4NH3��H2O==AlO2-+4NH4++2H2O
D������1 mol FeBr2��ˮ��Һ��ͨ���״����11.2 L Cl2��������Ӧ��Cl2+2Fe2+ == 2Fe3++2Cl-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�߶���9�µ��л�ѧ�Ծ��������棩 ���ͣ��ƶ���
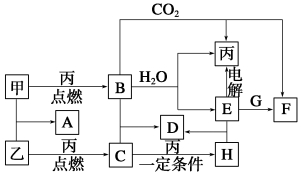
�ס��ҡ���Ϊ�������ʡ�A��B��C��D��E��F��G��H��Ϊ��ѧ��ѧ�г����Ļ��������B��G����ɫ��Ӧ��Ϊ��ɫ��C��ʹƷ����Һ��ɫ����һ�������£��������ת����ϵ��ͼ��ʾ��
��ش��������⣺
��1���û�ѧʽ��ʾ����Ϊ__________��HΪ__________��
��2��A�ĵ���ʽΪ___________________��
��3�����E��ˮ��Һʱ��E��������_____________________
��4��д��B��C�D��D�Ļ�ѧ����ʽ__________________________
д��E��G�D��F�����ӷ���ʽ_______________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�߶���9�µ��л�ѧ�Ծ��������棩 ���ͣ�ѡ����
�������ӷ���ʽ��ȷ����
A�����Ȼ�����Һ�м��������ˮ��Al3++3OH--=Al(OH)3��
B��4mol•L-1��NaAlO2��Һ��7mol•L-1��HCl�����������Ȼ�ϣ�4AlO2--+7H++H2O=3Al(OH)3��+Al3+
C��K37ClO3��Ũ����(HCl)�ڼ���ʱ����������K37ClO3+6HCl=K37Cl+3Cl2��+3H2O
D����25mL 0.l mol•L-1 ���Ỻ������25mL 0.1 mol•L-1Na2CO3��Һ�У������Ͻ��裺2H++CO32--=CO2��+H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�߶���9�µ��л�ѧ�Ծ��������棩 ���ͣ�ѡ����
Ӳ֬���������������Ӧ�õ�Ӳ֬���ƺ��͡��������Ӳ֬���ƣ�Ӧ���õķ����ǣ�������ֽ���� ���÷�Һ©����Һ �ۼ������� �ܼ����ȵı���ʳ��ˮ
A���ܢ� B���ܢ� C���٢� D���ܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�갲��ʡ��һ�Ͽ�ѧ�⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
�ס��ҡ������ֲ����ᾧˮ�Ĺ������ʵ��ܽ��������ͼ��ʾ������˵������ȷ����()

A��t2�� ʱ��50g�����ʷ���50gˮ�У�������Һ�����ʵ���������Ϊ50��
B��t1��ʱ�������ʵ��ܽ�ȴ�С��ϵ�ף��ң���
C�����������ļס��ҡ����������Ƴ�t2��ʱ�ı�����Һ����Ҫˮ���������ף��ң���
D�����������л��������ı����ʿɲ�ȡ���½ᾧ�ķ����ᴿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�갲��ʡ�߶��Ͽ�ѧ�⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
��������Ӱ�����ǵ������뽡����ij�����������п��ܺ������¿����������ӣ�Na+��NH4+��Mg2+��Al3+��SO42����NO3����Cl����ijͬѧ�ռ��˸õ���������������Ҫ��Ԥ������������Һ����Ʋ���������µ�ʵ�飺
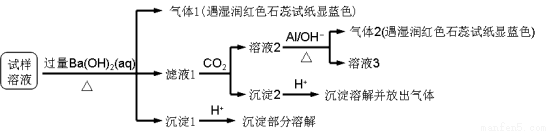
��֪��3NO3��+ 8Al + 5OH�� + 2H2O 3NH3��+ 8AlO2��
3NH3��+ 8AlO2��
�������ϵ�ʵ�����������ͬѧ�ó��Ľ��۲���ȷ����
A�������п϶�����NH4+��Mg2+��SO42����NO3��
B�������п��ܴ���Na+��Cl��
C��������һ������Al3+
D���������п��ܴ���NaNO3��NH4Cl��MgSO4
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com