
| ѡ�� | ʵ����������� | ʵ����� |
| A | ��ij��Һ�м��������ữ���Ȼ�����Һ���а�ɫ�������� | ����Һ��һ������SO |
| B | ��ij��Һ�м���2��KSCN��Һ����Һ����Ѫ��ɫ��������Һ�м��뼸�����Ƶ���ˮ����Һ��ΪѪ��ɫ | ����Һ��һ������Fe2�� |
| C | ij������ʹʪ�����ɫʯ����ֽ��� | ������ˮ��Һһ���Լ��� |
| D | ������ij�л���μӵ�����������ͭ����Һ�У������δ��ש��ɫ�������� | ���л��ﲻ��ȩ�� |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 ��SO
��SO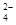 ���ӵ�ʵ�鷽����ȷ����________(����)��
���ӵ�ʵ�鷽����ȷ����________(����)���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | ʵ����� | ʵ������ | ���� |
| A | �����ݵ�������Һ�зֱ�μӱ���NaCl ��Һ��CuSO4��Һ | ���й������� | �����ʾ��������� |
| B | ����ҺX ���ȵμ�ϡ���ᣬ�ٵμ�Ba(NO3)2��Һ | ���ְ�ɫ���� | ��ҺX ��һ������SO42�� |
| C | ��һ��Ũ�ȵ�Na2SiO3��Һ��ͨ������CO2���� | ���ְ�ɫ���� | H2SiO3�����Ա�H2CO3������ǿ |
| D | ��Ũ�Ⱦ�Ϊ0. 1 mol��L-1 NaCl ��NaI �����Һ�еμ�����AgNO3��Һ | ���ֻ�ɫ���� | Ksp(AgCl)>Ksp(AgI) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

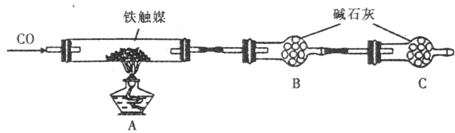
| ʵ����� | ʵ����������� |
| ȡ������ҺB���Թ��У��μ� ���� ������һ��ʱ��۲����� | ����Һ ������ҺB�к�Cl2�� ����Һ ������ҺB�в���Cl2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ��� | ������ | �����Լ� | ���뷽�� |
| A | CH4(C2H4) | ����KMnO4��Һ | ϴ�� |
| B | ������(Br2) | NaOH��Һ | ��Һ |
| C | �Ҵ�(����) | ����Na2CO3��Һ | ��Һ |
| D | C2H5OH(H2O) | ������ʯ�� | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
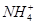 ��K����Na����Mg2����Ba2����Al3����Cl����I����
��K����Na����Mg2����Ba2����Al3����Cl����I����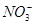 ��
��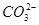 ��S2����
��S2����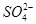 ��
��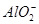 ��
��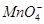 ��ȡ����Һ��������ʵ�飺
��ȡ����Һ��������ʵ�飺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��


| A��ͼ1�Ʊ����ռ�����NO2���� |
| B��ͼ2��ԭ���װ�ã�Fe�缫Ϊ���� |
| C��ͼ3�����ڷ��뻥�����ܵ�Һ�� |
| D��ͼ4������ʵ������ȡ���ռ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
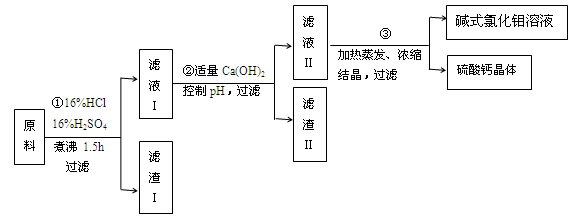
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

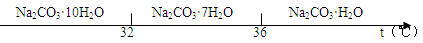
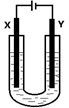
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com