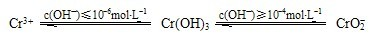��2012?��ƽ��һģ������Cr�������ؽ���Ԫ�أ�������ˮ�ͷ����ŷű��뾭�������ﵽ�йصİ�ȫ����
��1��������ˮ�ŷŵ���ˮ��һ��Ũ�Ȼ�ʹ�����ˮ������������ԭ����
�ؽ������ӻ�ʹ�����ʱ���
�ؽ������ӻ�ʹ�����ʱ���
��
���õĴ������������֣�
����1����ԭ������
�÷���FeSO
4����Cr
2O
72-����ʽ���������Է�ˮ�еĸ�Ԫ�ػ�ԭΪCr
3+�����ó��������з��룮��֪��
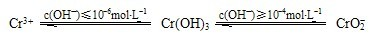
��2��FeSO
4��ԭCr
2O
72-�����ӷ���ʽΪ
Cr2O72-+6Fe2++14H+=3Cr3++6Fe3++7H2O
Cr2O72-+6Fe2++14H+=3Cr3++6Fe3++7H2O
��
��3��Cr
2��SO
4��
3��Һ�м������NaOHŨ��Һ����Ӧ�����ӷ���ʽΪ
Cr3++4OH-=CrO2-+2H2O
Cr3++4OH-=CrO2-+2H2O
��
��4�������������ˮ�е�Cr
3+���ӣ�pHӦ������
8��10
8��10
��Χ�ڣ�
��5�����й��ڸ����仯�����˵������ȷ����
abc
abc
��
a��K
2Cr
2O
7��-�ֳ��õ�ǿ������
b��NaCrO
2��Һ��AlCl
3��Һ����г�������
c������K
2Cr
2O
7��Һ�����ڼ���˾���Ƿ�ƺ�ݳ�
����2����ⷨ
�÷���Fe���缫��⺬Cr
2O
72-�����Է�ˮ�����ŵ����У�������������ҺpH���ߣ�����Cr��OH��
3������
��6����Fe���缫��ԭ��Ϊ
������ӦΪFe-2e-=Fe2+���ṩ��ԭ��Fe2+
������ӦΪFe-2e-=Fe2+���ṩ��ԭ��Fe2+
��
��7��������������ҺpH���ߵ�ԭ���ǣ��õ缫��Ӧ���ͣ�
2H++2e-=H2��
2H++2e-=H2��
����Һ��ͬʱ���ɵij�������
Fe��OH��3
Fe��OH��3
��