
(10 ��)����þ��ҽҩ����������ҵӦ�ù㷺������þ��ԭ�Ƚ��Ʊ��ߴ�����þ��һ���µ�̽��������þ��(��Ҫ�ɷ�ΪMgCO3��������FeCO3 )Ϊԭ���Ʊ��ߴ�����þ��ʵ���������£�
��1��MgCO3��ϡ���ᷴӦ�����ӷ���ʽΪ ��
��2������H2O2����ʱ��������Ӧ�Ļ�ѧ����ʽΪ ��
��3������2 �ijɷ��� (�ѧʽ)��
��4�����չ��̴������·�Ӧ��
2MgSO4+C 2MgO+2SO2��+CO2��
2MgO+2SO2��+CO2��
MgSO4+C MgO+SO2��+CO��
MgO+SO2��+CO��
MgSO4+3C MgO+S��+3CO��
MgO+S��+3CO��
������ͼװ�ö����ղ�����������зֲ����ջ��ռ���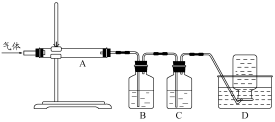
��D���ռ������������ (�ѧʽ)��
��B��ʢ�ŵ���Һ������ (����ĸ)��
a��NaOH ��Һ b��Na2CO3��Һ c��ϡ���� d��KMnO4��Һ
��A�еõ��ĵ���ɫ�������ȵ�NaOH��Һ��Ӧ��������Ԫ�����̬Ϊ+4��д���÷�Ӧ�����ӷ���ʽ�� ��
��1��MgCO3+2H��=Mg2��+CO2��+H2O (2��)
��2��2FeSO4+H2O2+H2SO4= Fe2(SO4)3+2H2O (2��)
��3��Fe(OH)3 (2��)
��4����CO (1��) ��d (1��) ��3S+6OH�� 2S2��+SO32��+3H2O (2��)
2S2��+SO32��+3H2O (2��)
��������������⻯ѧ����������Ҫע�������ͷ����ȷ����������ԭ�Ϻ�Ŀ�ģ��������̣���������Ϣ��ȷÿһ����Ŀ�ĺ�ԭ�����۴���Ҫע��淶��д��ѧ��������ֱ������������þ��(��Ҫ�ɷ�ΪMgCO3��������FeCO3 )Ϊԭ���Ʊ��ߴ�����þ�������������ܵ�����þ������������Һ�Ͳ��������Ϊ��ȥ������ù�������������������������Ϊ���������ð�ˮ��pH��������������ת������������������ȥ����������þ��Һ������������������þ����1��MgCO3��ϡ���ᷴӦ��������þ��ˮ�Ͷ�����̼�����ӷ���ʽΪMgCO3+2H��=Mg2��+CO2��+H2O����2������H2O2����ʱ����������������Ϊ��������������Ӧ�Ļ�ѧ����ʽΪ2FeSO4+H2O2+H2SO4= Fe2(SO4)3+2H2O����3������2 �ijɷ���Fe(OH)3����4�����������Ӧ֪������ijɷ�ΪSO2��CO2��CO��SO2���л�ԭ�ԣ�������KMnO4��Һ���գ�������������Һ���ն�����̼���������ˮ���ռ�CO��A�еõ��ĵ���ɫ����S���ȵ�NaOH��Һ��Ӧ��Ӧ�����ӷ���ʽΪ��3S+6OH�� 2S2��+SO32��+3H2O����Ϊ����CO ��d ��3S+6OH��
2S2��+SO32��+3H2O��������CO ��d ��3S+6OH�� 2S2��+SO32��+3H2O��
2S2��+SO32��+3H2O��
���㣺�Ի�ѧ��������Ϊ���壬���黯ѧ����ʽ����д�����ʵķ��롣


 ��У���˿��ֿ���ϵ�д�
��У���˿��ֿ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����пΪ��ɫ��ĩ��������ʪ�Ѣ��Ƥ���������ơ�������ҵ������п[����Fe(��)��Mn(��)��Ni(��)������]���������£�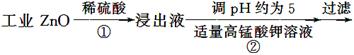
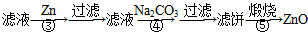
��ʾ���ڱ�ʵ�������£�Ni(��)���ܱ�������������صĻ�ԭ������MnO2��
�ش��������⣺
(1)��Ӧ���г���������������__________��������Ӧ�����ӷ���ʽΪ__________���ڼӸ��������Һǰ����pH�ϵͣ��Գ��ӵ�Ӱ����________________��
(2)��Ӧ�۵ķ�Ӧ����Ϊ____________�����˵õ��������У����˹�����п���______________��
(3)��Ӧ���γɵij���Ҫ��ˮϴ����������Ƿ�ϴ�Ӹɾ��ķ�����________________��
(4)��Ӧ���в���ijɷֿ�����ZnCO3��xZn(OH)2��ȡ�������˱�11.2 g�����պ�ɵõ���Ʒ8.1 g����x����__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������㷺Ӧ������ҩ�ͻ�����ҵ��ij��ѧС���üױ�����Ҫԭ���Ʊ������ᣬ��Ӧ�������£�
�ױ���������ء�������IJ����������ʼ��±���
| ���� | �۵�/�� | �е�/�� | �ܶ�/g��cm-3 | ��ˮ���ܽ��� |
| �ױ� | ��95 | 110.6 | 0.8669 | ���� |
| ������� | 121.5��123.5 |  |  | ���� |
| ������ | 122.4 | 248 | 1.2659 | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ϸ���������Ҫ�ɷ���V2O5��VOSO4��K2SO4��SiO2��Fe2O3�ȣ������������¹������̻���V2O5��
�ش��������⣺
��1�� VOSO4�У�VԪ�صĻ��ϼ�Ϊ_______�����в����ķ�������Ҫ�ɷ���_________��
��2����ƽ���з�Ӧ�����ӷ���ʽ��
��3��25��ʱ��ȡ������ʵ��������õ��������ʺ���ҺpH֮��Ĺ�ϵ���±���
���ж���ʵ������ʱ�����м��백ˮ������Һ�����pHΪ______________��
��4������ʱ�������е���������Һѭ�����ڢ��е�ˮ�������������������У���ѭ�����õ����ʻ���________________��
��5����ƷV2O5��ͨ�����ȷ�Ӧ����ȡ��������д���÷�Ӧ�Ļ�ѧ����ʽ��______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ʯ����Կ�����MgO��Fe2O3��Al2O3��SiO2��ɣ�������ʯ��ȡ��ʽ̼��þ��ʵ�鲽�����£�
��1������ʯ��������ܽ����Һ�����Mg2+�⣬�����еĽ��������� ��
��2�����Т����ʱ��������Һ��pH=7~8���й��������������pH���±�����Ca(OH)2���ܹ�������Ca(OH)2�������ܻᵼ�� �ܽ⣬���� ������
| �������� | Fe(OH)3 | Al(OH)3 | Mg(OH)2 |
| ��ʼ����pH | 1.5 | 3.3 | 9.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ᴿ����ʯ(��Ҫ������������)������ͼ��
��Ҫ��ش��������⣺
��1���ڹ�ҵ�����У�����״����ʯĥ�ɷ�ĩ�����ڷ�Ӧ���а�װ�������Ŀ����________������ҺB�õ�����B���������̰���___________(���������)����ȴ�ᾧ��
��2����������A�������Ӧѡ��___________(�ѧʽ)���ڸù�����Ҫ��ʱ�ⶨpH����ʵ��������pH��ֽ�ⶨ��ҺpH�IJ�����______________________________��
��3������Fe3+����ȫ����Fe(OH)3�����ķ�����________________________________��
��4��д������ʯ�ܽ�ʱ��������Ӧ�����ӷ���ʽ_______________;_________________.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������棨CeO2����һ����Ҫ��ϡ�������ƽ�������ʾ�����������в��������ķϲ�����ĩ����SiO2��Fe2O3��CeO2�����ʣ���ij�����Դ˷�ĩΪԭ�ϣ���Դ���յĹ����������£�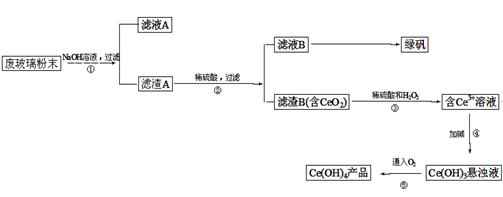
��1��д���ڢٲ���Ӧ�����ӷ���ʽ ��
��2��ϴ������B��Ŀ����Ϊ�˳�ȥ____�������ӷ��ţ�������������Ƿ�ϴ���ķ����� ��
��3��д���ڢ۲���Ӧ�Ļ�ѧ����ʽ_____________________________��
��4���Ʊ��̷�(FeSO4��7H2O)ʱ����Fe2(SO4)3��Һ�м��������м����ַ�Ӧ�����˵õ�FeSO4��Һ���پ� �� �����ˡ�ϴ�ӡ�����Ȳ�������õ��̷���
��5��ȡ���������еõ���Ce(OH)4��Ʒ(��������Ϊ97%)1.0g���������ܽ����0.1000mol/LFeSO4��Һ�ζ����յ㣨�汻��ԭ��Ce3+)������ȷ�μӱ���Һ�����Ϊ mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ƣ�NaClO2����һ����Ҫ�ĺ�������������Ҫ���ڹ�ҵ������Ư��������
��ͼ�ǹ������ⷨ�����������ƵĹ�������ͼ��
������ʵ��������£�
�� ClO2�е����������Ũ�Ƚϸߵ�ClO2�����ֽⱬը��
�� NaClO2�ܽ�����¶����߶������ʵ������¿ɽᾧ����NaClO2��3H2O��
��1��.��ClO2��������ͨ��SO2��ͬʱ�����������������________(�����)��
A����SO2������SO3����ǿ����
B��ϡ��ClO2�Է�ֹ��ը
C����NaClO3������ClO2
��2��Ϊȷ��H2O2��ֲ��뷴Ӧ�����������¶Ȳ��˽ϸߣ���ԭ����______________��
��3��160 g��L-1 NaOH��Һ�����ʵ���Ũ��Ϊ______________����ʵ������Ҫ����450mL�����ʵ���Ũ��NaOH��Һ��Ӧ��ȡNaOH������Ϊ__________�ˡ�
��4��д���������ڷ�Ӧ�Ļ�ѧ����ʽ_____________________________________��
��5������Һ�еõ�NaClO2��3H2O�־����ʵ�����������____________(�����)��
A������ B������ C������ D������ E����ȴ�ᾧ
Ҫ�õ�������NaClO2��3H2O��������һ���IJ�����________(���������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
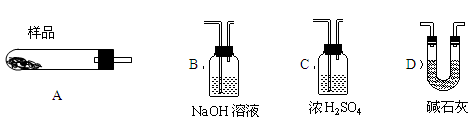
�ӻ��յĺ�ͭ���·�������ȡͭʱ����������������ַ������ش��й����⡣
�����ף�
�����ң�
��1�����������У����ϵ�ǰ��������ɫ��ѧ������Ƿ��� �������� ������һ�������ա��������Ⱦ���������塢�۳���������
��2����������ͭ�ܽ�������ɫ��Һʱ������Ӧ�����ӷ���ʽΪ________________ _ ______��
��3��Ϊ�����ԭ�ϵ�������,���һ������dz��ɫ��Һͨ������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ���Ȼ����ɵõ�һ�ֽᾧˮ����ľ��塣��þ���������м�⣺
����ȡa g�ľ��������ˮʵ�飬�����ˮ����Ϊ��a��1.26��g
�ڽ���ˮ��������������ˮ�����Һ��μ�1.00mol/L���Ȼ�����Һ�����μ�10.00mL��Һʱ������ǡ����ȫ��ͨ�������֪�þ���Ļ�ѧʽ�� ��
��4���Ȼ���ͭ��CuCl������Ҫ�Ļ���ԭ�ϡ����ұ��涨�ϸ��CuCl��Ʒ����Ҫ����ָ��ΪCuCl��������������96.5% ����ҵ��������ͭ��ԭ�ϳ�ͨ�����з�Ӧ�Ʊ�CuCl ��
2CuSO4+ Na2SO3 + 2 NaCl + Na2CO3��2 CuCl ��+ 3 Na2SO4 + CO2��
�ⶨCuCl��������ʱ��ȷ��ȡ���Ʊ���0.2500g CuCl��Ʒ����һ������0.5mol��L-1 FeCl3��Һ�У�����Ʒ��ȫ�ܽ��ˮ20mL����0.1000mol��L-1 Ce��SO4��2��Һ�ζ����յ㣬����24.60mL Ce��SO4��2��Һ���йط�Ӧ�����ӷ���ʽΪ��Fe 3++CuCl��Fe 2++Cu2++Cl�� ��Ce4+ + Fe 2+��Fe 3+ + Ce3+
����������Ʒ��CuCl����������Ϊ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com