
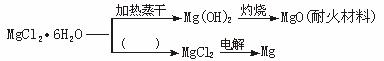
Зл»ШөрПВБРОКМвӘғ
ӘЁ1Ә©ФЪНәЦРµДАЁғЕДЪМоРөККµ±µД·өУ¦МхәюҰӘ
ӘЁ2Ә©MgӘЁOHӘ©2№ММеөжФЪИзПВИЬҢвЖҢғвӘғ
MgӘЁOHӘ©2ӘЁsӘ©![]() Mg2Ә«Ә«2OHӘҰӘПтМеПµЦРәУИлӘЁЦБЙЩМоБҢЦЦІ»Н¬Аа±рµДОпЦКӘ©______________Ә¬УРАыУЪMgӘЁOHӘ©2ИЬҢвҰӘ
Mg2Ә«Ә«2OHӘҰӘПтМеПµЦРәУИлӘЁЦБЙЩМоБҢЦЦІ»Н¬Аа±рµДОпЦКӘ©______________Ә¬УРАыУЪMgӘЁOHӘ©2ИЬҢвҰӘ
ӘЁ3Ә©NaFғНMgOµДғЛәдңаАл·Ц±рОҒ2.31ҰБ10Ә10 mғН2.10ҰБ10Ә10 mӘ¬µ«БҢХЯµДИЫµг·Ц±рОҒ993 ҰжғН2852 ҰжҰӘКФҢвКНЖдүЙДЬµДФТтҰӘ
ӘЁ4Ә©ТАңЭВМЙ«»ҮС§µДФЧУңәГµДёЕДоӘЁәө»ҮС§·өУ¦µДФБПЦРГүёцФЧУ¶әІОУл·өУ¦ІұИ«ІүЧҒ»ҮОҒІъОпӘ©Ә¬203 kg MgCl2Ұ¤6H2OФБПӘ¬үЙТФ»сµГ28.8 kg MgOҰұ________kg 36.5%µДСОЛбғН________kg MgCl2ҰӘ
ҢвОцӘғnӘЁMgOӘ©ӘҢ![]() ӘҢ745 mol nӘЁMgCl2Ә©ӘҢ
ӘҢ745 mol nӘЁMgCl2Ә©ӘҢ![]() Ә745ӘҢ255 mol
Ә745ӘҢ255 mol
әөmӘЁMgCl2Ә©ӘҢ255ҰБ95ӘҢ24225 gӘҢ24.225 kg
ңЭЦКБүКШғгӘғ36.5%µДСОЛбЦКБүӘҢ203Ә29.8Ә24.2ӘҢ149 kgҰӘ
өр°ёӘғӘЁ1Ә©ФЪёЙФпµДВИ»ҮЗвЖшБчЦРәУИИ
ӘЁ2Ә©ЛбАаӘғHClҰұH2SO4ҰұHNO3µИӘ»ЗүЛбИхәоСОӘғCuSO4ҰұFeCl3µИӘ»п§СОАаӘғCH3COONH4ҰұNH4ClµИӘЁЦ»ТҒРөіцБҢАаәөүЙӘ©
ӘЁ3Ә©БҢЦЦОпЦКН¬КЗАлЧУң§МеӘ¬MgO±ИNaFәьі¤¶МӘЁ»тMgO±ИNaFғЛәдңаАлРҰӘ©Ә¬Mg2Ә«ҰұO2ӘЛщөшµзғЙКэ¶аӘЁАлЧУЦ®әдЧчУГәУөуӘ©Ә¬АлЧУң§МеµДИЫµгТІПФЦшЙэёЯҰӘ
ӘЁ4Ә©149 24.2


 ҢрФүіЧКФңнПµБРөр°ё
ҢрФүіЧКФңнПµБРөр°ё
| Дкә¶ | ёЯЦРүОіМ | Дкә¶ | іхЦРүОіМ |
| ёЯТ» | ёЯТ»Гв·СүОіМНЖәцӘҰ | іхТ» | іхТ»Гв·СүОіМНЖәцӘҰ |
| ёЯ¶ю | ёЯ¶юГв·СүОіМНЖәцӘҰ | іх¶ю | іх¶юГв·СүОіМНЖәцӘҰ |
| ёЯИэ | ёЯИэГв·СүОіМНЖәцӘҰ | іхИэ | іхИэГв·СүОіМНЖәцӘҰ |
үЖДүӘғёЯЦР»ҮС§ АөФөӘғ МвРНӘғ


Ійүөөр°ёғНҢвОц>>
№ъәКС§РӘУЕСҰ - Б·П°ІбБР±н - КФМвБР±н
ғю±±КҰ»ӨБҒНшОӨ·ЁғНІ»БәРЕПұңЩ±ЁЖҢМЁ | НшЙПУРғ¦РЕПұңЩ±ЁЧЁЗш | µзРЕХ©ЖңЩ±ЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРғ¦РЕПұңЩ±ЁЧЁЗш | ЙжЖуЗЦИЁңЩ±ЁЧЁЗш
ОӨ·ЁғНІ»БәРЕПұңЩ±Ёµз»°Әғ027-86699610 ңЩ±ЁУКПдӘғ58377363@163.com