
��6�֣�����ͼ��ʾ��װ��A�У���KMnO4�����Ũ��������ȡCl2����Ӧ�����ӷ���ʽΪ��2MnO4��+10Cl��+16H+=2Mn2++5Cl2��+8H2O��װ��B�е��Ĵ��������������´�������պ�е��ۡ�KI��Һ ��պ��Ʒ����Һ��պ����ɫʯ����Һ��պ��ŨNaOH��Һ����ͼ�мг�װ������ȥ����

�ش��������⣺
��1��p��ʢװ�Լ�������Ϊ ��
��2����Ӧ��ʼ�۲쵽��ʵ�������ǣ�
�ٴ��� ���ڴ��� ��
�۴��� ��
��3��д���ܴ���Ӧ�����ӷ�ʽ�� ��
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����ȼ�ϵ���Ƿ�����ɫ��ѧ��������ͷ���װ�ã���ͼΪ���ʾ��ͼ���õ�ص缫�����һ��ϸС�IJ��ۣ����������������ǿ�������ȶ�����ش�
����ȼ�ϵ���Ƿ�����ɫ��ѧ��������ͷ���װ�ã���ͼΪ���ʾ��ͼ���õ�ص缫�����һ��ϸС�IJ��ۣ����������������ǿ�������ȶ�����ش�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
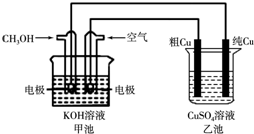 ��ͭ��һ�㺬��п����������������ʣ�����ͼ��ʾ��װ���У��׳ص��ܷ�Ӧ����ʽΪ��2CH3OH+3O2+4KOH�T2K2CO3+6H2O ��ͨ��·һ��ʱ���Cu�缫����������3.2g���ڴ˹����У�����˵����ȷ���ǣ�������
��ͭ��һ�㺬��п����������������ʣ�����ͼ��ʾ��װ���У��׳ص��ܷ�Ӧ����ʽΪ��2CH3OH+3O2+4KOH�T2K2CO3+6H2O ��ͨ��·һ��ʱ���Cu�缫����������3.2g���ڴ˹����У�����˵����ȷ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��6�֣�����ͼ��ʾ��װ��A�У���KMnO4�����Ũ��������ȡCl2����Ӧ�����ӷ���ʽΪ��2MnO4��+10Cl��+16H+=2Mn2++5Cl2��+8H2O��װ��B�е��Ĵ��������������´�������պ�е��ۡ�KI��Һ ��պ��Ʒ����Һ��պ����ɫʯ����Һ��պ��ŨNaOH��Һ����ͼ�мг�װ������ȥ����
�ش��������⣺
��1��p��ʢװ�Լ�������Ϊ ��
��2����Ӧ��ʼ�۲쵽��ʵ�������ǣ�
�ٴ��� ���ڴ��� ��
�۴��� ��
��3��д���ܴ���Ӧ�����ӷ�ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꼪��ʡ����һ�и߶���ѧ��������⻯ѧ�Ծ� ���ͣ������
��6�֣�����ͼ��ʾ��װ��A�У���KMnO4�����Ũ��������ȡCl2����Ӧ�����ӷ���ʽΪ��2MnO4��+10Cl��+16H+=2Mn2++5Cl2��+8H2O��װ��B�е��Ĵ��������������´�������պ�е��ۡ�KI��Һ��պ��Ʒ����Һ��պ����ɫʯ����Һ��պ��ŨNaOH��Һ����ͼ�мг�װ������ȥ����
�ش��������⣺
��1��p��ʢװ�Լ�������Ϊ ��
��2����Ӧ��ʼ�۲쵽��ʵ�������ǣ�
�ٴ��� ��
�ڴ��� ��
�۴��� ��
��3��д���ܴ���Ӧ�����ӷ�ʽ�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com