


 ����Ϊƽ���������νṹ��
����Ϊƽ���������νṹ�� ��ƽ���������Σ�
��ƽ���������Σ�


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)�����ӵĵ���ʽ��____________�������ӵĿռ乹��Ϊ_____________��
(2)����������ڿ�����Ⱦ��һ����ʩ��________________________________________��
(3)�ҹ������ڿ������������涨���ڿ����м�ȩ�������ó���0.08 mg��m-3����ȩ�ⶨ�ķ�����Ҫ�зֹ��ȷ���ɫ�����绯ѧ���ͻ�ѧ�ζ����ȡ�ijУ�о���ѧϰС���ͬѧ����û�ѧ�ζ����Խ����ڿ����м�ȩ�ĺ������вⶨ������������о����⣬��ɼ���ȩ��Ʒ�ķ�����________________________________________________________________��
(4)�������ǻ�ѧ�ζ���֮һ��������ԭ��Ϊ�ڼ��Խ���(NaOH)�У���ת��Ϊ�ε����ƺ͵⻯�ƣ��ε����ƽ���Һ������ļ�ȩ����Ϊ�����ƣ����ʵ��ữ��ʣ��Ĵε�������⻯�������ɵ⣻�Ե���Ϊָʾ��������������Ʊ���Һ�ζ��������������漰�Ļ�ѧ��Ӧ�����ӷ���ʽ����ΪI2+2OH-![]() IO-+I-+H2O��___________________________________��____________________________��I2+2
IO-+I-+H2O��___________________________________��____________________________��I2+2![]()
![]()
![]() +2I-��ʵ���������Ҫ�ⶨ�����ݳ��ܵ����⣬����_____________��
+2I-��ʵ���������Ҫ�ⶨ�����ݳ��ܵ����⣬����_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������ճ�����������Ҫ�ij������ִ����ڡ�ú���͡������⻯ѧ��������Ⱦ�����ڽ����ԡ����ڿ�����Ⱦ��Ϊ��־�ĵ�����Ⱦʱ�ڡ�������ڻ�����Ⱦ���ж�������Ҫ�м�ȩ��������������֡�
��ش��������⣺
��1�������ӵĵ���ʽ�� ?? �������ӵĿռ乹��Ϊ ?? ��
��2������������ڿ�����Ⱦ��һ����ʩ�� ?? ��
��3���ҹ������ڿ������������涨���ڿ����м�ȩ�������ó���0.08mg??m-3����ȩ�ⶨ�ķ�����Ҫ�зֹ��ȷ���ɫ�����绯ѧ������ѧ�ζ����ȡ�ijУ�о���ѧϰС���ͬѧ����û�ѧ�ζ����Խ����ڿ����м�ȩ�ĺ������вⶨ������������о����⣬��ɼ���ȩ��Ʒ�ķ����� ?? ��
��4���������ǻ�ѧ�ζ���֮һ��������ԭ��Ϊ�ڼ��Խ��ʣ�NaOH���У���ת��Ϊ�ε����ƺ͵⻯�ƣ��ε����ƽ���Һ������ļ�ȩ����Ϊ�����ƣ����ʵ��ữ��ʣ��Ĵε�������⻯�������ɵ⣻�Ե���Ϊָʾ��������������Ʊ���Һ�ζ��������������漰�Ļ�ѧ��Ӧ�����ӷ���ʽ����ΪI2+2OH-====IO-+I-+H2O��
?? �� ?? ��I2+2S2O32-====S4O62-+2I-��ʵ���������Ҫ�ⶨ�����ݳ��ܵ����⣬���� ?? ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡͬ���� ���ͣ������
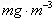 �ⶨ��ȩ�ķ�����Ҫ�зֹ��ȷ���ɫ�����绯ѧ������ѧ�ζ����ȡ�ijУ�о���ѧϰС���ͬѧ����û�ѧ�ζ����Խ����ڿ����м�ȩ�ĺ������вⶨ,����������о�����,��ɼ���ȩ��Ʒ�ķ�����______________________��
�ⶨ��ȩ�ķ�����Ҫ�зֹ��ȷ���ɫ�����绯ѧ������ѧ�ζ����ȡ�ijУ�о���ѧϰС���ͬѧ����û�ѧ�ζ����Խ����ڿ����м�ȩ�ĺ������вⶨ,����������о�����,��ɼ���ȩ��Ʒ�ķ�����______________________�� 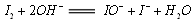 ����___________________�� ��________________��
����___________________�� ��________________��  ��
�� �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣������������ճ�����������Ҫ�ij������ִ����ڡ�ú���͡������⻯ѧ��������Ⱦ�����ڽ����ԡ����ڿ�����Ⱦ��Ϊ��־�ĵ�����Ⱦʱ�ڡ�������ڻ�����Ⱦ���ж�������Ҫ�м�ȩ��������������֡�
��ش��������⣺
��1�������ӵĵ���ʽ�� �������ӵĿռ乹��Ϊ ��
��2������������ڿ�����Ⱦ��һ����ʩ�� ��
��3���ҹ������ڿ������������涨���ڿ����м�ȩ�������ó���0.08mg•m-3����ȩ�ⶨ�ķ�����Ҫ�зֹ��ȷ���ɫ�����绯ѧ������ѧ�ζ����ȡ�ijУ�о���ѧϰС���ͬѧ����û�ѧ�ζ����Խ����ڿ����м�ȩ�ĺ������вⶨ������������о����⣬��ɼ���ȩ��Ʒ�ķ����� ��
��4���������ǻ�ѧ�ζ���֮һ��������ԭ��Ϊ�ڼ��Խ��ʣ�NaOH���У���ת��Ϊ�ε����ƺ͵⻯�ƣ��ε����ƽ���Һ������ļ�ȩ����Ϊ�����ƣ����ʵ��ữ��ʣ��Ĵε�������⻯�������ɵ⣻�Ե���Ϊָʾ��������������Ʊ���Һ�ζ���
�����������漰�Ļ�ѧ��Ӧ�����ӷ���ʽ����Ϊ: ��I2+2OH��====IO�� + I�� + H2O ���� ���� ����I2+2S2O32��====S4O62��+2I����
ʵ���������Ҫ�ⶨ�����ݳ��ܵ����⣬���� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com