
����ԭ��X��ͨ���������̺ϳɣ�
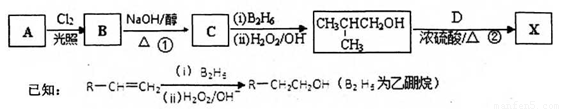
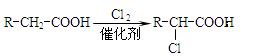
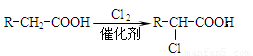
(1)��A����Է���������58����A�ķ���ʽ�� ��B��A��һ��ȡ���������ӵĺ˴Ź�������ֻ��һ�ַ壬д��B�����ƣ�ϵͳ������ ��
(2)��Ӧ�ٵĻ�ѧ����ʽΪ
(3)����D (C9H8O2)�Ľṹ�к��б������ұ�����һ�ȴ���ֻ�����֣�D����NaHCO3 ��Һ��Ӧ����CO2����Ӧ�ڵĻ�ѧ����ʽΪ ��
(4)д�����ַ�������������D��ͬ���칹�壺_______��_______���ṹ��ʽ)��
a����FeC3��Һ����ɫ b���ܷ���������Ӧ c��������������ȡ����
(5)���й���X���ʵ�˵���У���ȷ����_______��
a��������ˮ���е�ϵ�
b���ܷ����ӳɷ�Ӧ��ȡ����Ӧ
c����ʹ���Ը��������Һ��ɫ
d��1 mol X����Һ������ܺ�2 mol NaOH��Ӧ
����16�֣�
��1��C4H10 ��2�֣� 2������2���ȱ��飨2�֣�
��2��(CH3)3CCl+NaOH (CH3)2C=CH2��+NaCl+H2O
��3�֣�
(CH3)2C=CH2��+NaCl+H2O
��3�֣�
��3�� ��3�֣�
��3�֣�
��4��






����4�֣�д�������𰸼��ɣ�
��5��b.c��2�֣�
��������
�����������A����Է�������Ϊ58��C�Ľṹ����ȷ��A�ķ���ʽΪC4H10���ṹΪ(CH3)2CHCH3��A����һȡ��������B����B�ĺ˴Ź�������ֻ��һ��壬�Ӷ�ȷ��B�ĽṹΪ(CH3)2CClCH3������Ϊ2-��-2-��-���飬����NaOH�Ĵ���Һ�з�����ȥ����C��C�ĽṹΪ(CH3)2C=CH2��Ȼ���������顢˫��ˮ�����·�����������2-��-1-������D�Ľṹ�к��б������ұ�����һ�ȴ���ֻ�����֣�D����NaHCO3 ��Һ��Ӧ����CO2����֪D�ĽṹΪ ���ʷ�Ӧ��Ϊ������Ӧ���Ӷ��õ�X��X�к��б�����̼̼˫�������������ܷ����ܷ���ȡ�����ӳɡ�������Ӧ��������ֻ�ܺ�1molNaOH������Ӧ��
���ʷ�Ӧ��Ϊ������Ӧ���Ӷ��õ�X��X�к��б�����̼̼˫�������������ܷ����ܷ���ȡ�����ӳɡ�������Ӧ��������ֻ�ܺ�1molNaOH������Ӧ��
���㣺���л��ƶ�Ϊ�����������л�������ŵ����ʼ�ת�����л���Ӧ�����ͣ��л���ͬ���칹�����д�����֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ҫ�Ļ���ԭ��F��C5H8O4�����������ζ����ͨ���������̺ϳɣ�
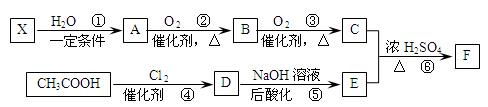
��֪����X��ʯ���ѽ�����Ҫ�ɷ�֮һ������ϩ��Ϊͬϵ�
��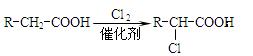 ��
��
��C��E��F������NaHCO3��Ӧ�������塣
��1��X�Ӿ۲���Ľṹ��ʽ�� ��
��2��D�����������ŵ������� ��
��3����Ӧ�ڵĻ�ѧ����ʽΪ ����Ӧ������ ��
��Ӧ�Ļ�ѧ����ʽΪ ��
��4��F��ͬ���칹��ܶ࣬����һ��ͬ���칹��ֻ����һ�ֹ����ţ������Ի���������¶���ˮ�����������л����ͬ���칹��Ľṹ��ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ҫ�Ļ���ԭ��F��C5H8O4�����������ζ����ͨ���������̺ϳɣ�
��֪����X��ʯ���ѽ�����Ҫ�ɷ�֮һ������ϩ��Ϊͬϵ�
����
��C��E��F������NaHCO3��Ӧ�������塣
��1��X�Ӿ۲���Ľṹ��ʽ�� ��
��2��D�����������ŵ������� ��
��3����Ӧ�ڵĻ�ѧ����ʽΪ ����Ӧ������ ��
��Ӧ�Ļ�ѧ����ʽΪ ��
��4��F��ͬ���칹��ܶ࣬����һ��ͬ���칹��ֻ����һ�ֹ����ţ������Ի���������¶���ˮ�����������л����ͬ���칹��Ľṹ��ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�����ʡ����һ�и�����ѧ����ĩ���ԣ����ۣ���ѧ���� ���ͣ������
��Ҫ�Ļ���ԭ��F��C5H8O4�����������ζ����ͨ���������̺ϳɣ�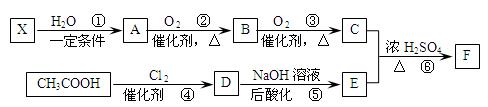
��֪����X��ʯ���ѽ�����Ҫ�ɷ�֮һ������ϩ��Ϊͬϵ�
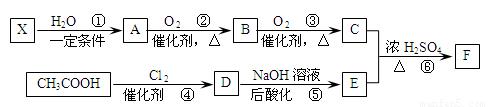
�� ��
��
��C��E��F������NaHCO3��Ӧ�������塣
��1��X�Ӿ۲���Ľṹ��ʽ�� ��
��2��D�����������ŵ������� ��
��3����Ӧ�ڵĻ�ѧ����ʽΪ ����Ӧ������ ��
��Ӧ�Ļ�ѧ����ʽΪ ��
��4��F��ͬ���칹��ܶ࣬����һ��ͬ���칹��ֻ����һ�ֹ����ţ������Ի���������¶���ˮ�����������л����ͬ���칹��Ľṹ��ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�����ʡ������ѧ����ĩ���ԣ����ۣ���ѧ���� ���ͣ������
��Ҫ�Ļ���ԭ��F��C5H8O4�����������ζ����ͨ���������̺ϳɣ�
��֪����X��ʯ���ѽ�����Ҫ�ɷ�֮һ������ϩ��Ϊͬϵ�
�� ��
��
��C��E��F������NaHCO3��Ӧ�������塣
��1��X�Ӿ۲���Ľṹ��ʽ�� ��
��2��D�����������ŵ������� ��
��3����Ӧ�ڵĻ�ѧ����ʽΪ ����Ӧ������ ��
��Ӧ�Ļ�ѧ����ʽΪ ��
��4��F��ͬ���칹��ܶ࣬����һ��ͬ���칹��ֻ����һ�ֹ����ţ������Ի���������¶���ˮ�����������л����ͬ���칹��Ľṹ��ʽ�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com