
��10�֣�ÿ��2�֣��ס�������ʵ��С������KMnO4������Һ��H2C2O4��Һ��Ӧ�о�Ӱ�췴Ӧ���ʵ����ء�
��1���÷�Ӧ�����ӷ���ʽΪ����ʾ��H2C2O4��һ������ƽ�ⳣ��Ϊ5.4��10-2��

 ��
��
���ʵ�鷽�����£�ʵ��������KMnO4��Һ���Ѽ���H2SO4����
��2�����飺ͨ���ⶨ��λʱ��������CO2��������Ĵ�С���Ƚϻ�ѧ��Ӧ���ʵĴ�С��ʵ��װ����ͼ��ʵ��ʱ��Һ©����A��Һһ���Է��£�A��B�ijɷּ��±���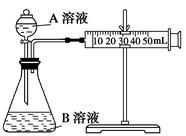
| ��� | A��Һ | B��Һ |
| �� | 2 mL 0.1 mol/L H2C2O4��Һ | 4 mL 0.01 mol/L KMnO4��Һ |
| �� | 2 mL 0.2 mol/L H2C2O4��Һ | 4 mL 0.01 mol/L KMnO4��Һ |
| �� | 2 mL 0.2 mol/L H2C2O4��Һ | 4 mL 0.01 mol/L KMnO4��Һ������MnSO4 |
 mol/L
mol/L H2C2O4��Һ����ȡ��֧�Թܸ�����4 mL 0.1 mol/L KMnO4��Һ������֧�Թֳܷ����飨����һ֧ʢ��H2C2O4��Һ��KMnO4��Һ���Թܣ���һ�������ˮ�У���һ�������ˮ�У�����һ��ʱ��ֱ���
H2C2O4��Һ����ȡ��֧�Թܸ�����4 mL 0.1 mol/L KMnO4��Һ������֧�Թֳܷ����飨����һ֧ʢ��H2C2O4��Һ��KMnO4��Һ���Թܣ���һ�������ˮ�У���һ�������ˮ�У�����һ��ʱ��ֱ��� ������¼��Һ��ɫ����ʱ�䡣��ʵ��Ŀ�����о� �Ի�ѧ��Ӧ���ʵ�Ӱ�죬������ͬѧʼ��û�п�����Һ��ɫ����ԭ���� ��
������¼��Һ��ɫ����ʱ�䡣��ʵ��Ŀ�����о� �Ի�ѧ��Ӧ���ʵ�Ӱ�죬������ͬѧʼ��û�п�����Һ��ɫ����ԭ���� ��  ����ѧУ�ֲ����ܲ�ϵ�д�
����ѧУ�ֲ����ܲ�ϵ�д� �ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
�ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2010������ʡ������У������߶���ѧ�����п��Ի�ѧ�� ���ͣ���ѡ��
��10�֣�ÿ��2�֣����ݻ�Ϊ1L���ܱ������У��������·�Ӧ��A��g��+2B��g�� C��g��+D��g�����ڲ�ͬ�¶��£�D�����ʵ���n��D����ʱ��t�Ĺ�ϵ��ͼ���Իش��������⣺
C��g��+D��g�����ڲ�ͬ�¶��£�D�����ʵ���n��D����ʱ��t�Ĺ�ϵ��ͼ���Իش��������⣺
��1��800��ʱ��0��5min�ڣ���B��ʾ��ƽ����Ӧ����Ϊ ��
��2�����жϸ÷�Ӧ�ﵽ��ѧƽ��״̬�������� ��
����A��������ѹǿ���� B�����������c��A������
����C��2v����B��=v����D�� D��c��A��=c��C��
��3�����������1.0molA��2.2molB������ͼ�����ݼ���800��ʱ��ƽ�ⳣ��K= ���÷�ӦΪ ��Ӧ�������Ȼ���ȣ�
��4��700��ʱ��ijʱ�̲����ϵ�и����ʵ������£�n��A��=1.1mol��n��B��=2.6mol��n��C��=0.9mol��n��D��=0.9mol�����ʱ�÷�Ӧ ���У��������Ӧ�������淴Ӧ������ƽ��״̬������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015��㶫ʡ�����и�һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
(10�֣�ÿ��2��)
Ư�ۿ��Ժ�Ũ���ᷴӦ����������ij������ȤС����ͼ�ⶨ�������������֤�������������û��Ư���ԣ�������ͼ��ʾװ�ý���ʵ�飬��ش��й����⣺

��1����װ�õ���ȷ����˳���ǣ�a��( )��( )��( )��( )��( )��( )��( )��( )��( )��
��2��U�ܿ�������ʢװ__________��ϴ��ƿ����
��3������ȡ����ǰ��������е�һ�����������__________________
��4������ʵ��Ŀ�Ľ���֮������ɫ�����Ƿ���ɫ( )(���ǻ��)��
��5������ȤС����ʵ���У�������Ͳ�в�û���ռ���Һ�壬����Ϊ����ʧ�ܵ�ԭ�������____________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ����8���¿���ѧ�Ծ��������棩 ���ͣ������
��10�֣�ÿ��2�֣������������ʵ���Ҫ���������ʵĽṹ����ش��������⣺
(1)��֪A��BΪ��������Ԫ�أ���ԭ�ӵĵ�һ�����ĵ������±���ʾ��
|
������/kJ��mol��1 |
I1 |
I2 |
I3 |
I4 |
|
A |
578 |
1 817 |
2 745 |
11 578 |
|
B |
738 |
1 451 |
7 733 |
10 540 |
Aͨ����____�ۣ�A�ĵ縺��__ __B�ĵ縺��(�����������������)��
(2)��֪������Ϊ300 nm�������Ĺ��������е�����ԼΪ399 kJ��mol��1�������±��йص����ʷ�������Ҫ��ѧ������Ϣ��˵�����峤ʱ������������Ƥ�������˺���ԭ��
��
|
���ۼ� |
C��C |
C��N |
C��S |
|
����/kJ��mol��1 |
347 |
305 |
259 |
(3)�о����ʴ��Ա��������������Ӻ�δ�ɶԵ���Խ�࣬�����Խ�ż�¼����Խ�á�������������V2O5��CrO2�У��ʺ���¼�����ŷ�ԭ�ϵ���________________��
(4)ij�����ķ��ӽṹ��ͼ��ʾ��������ڲ�����__________(����ĸ)��
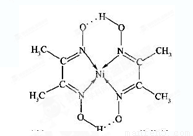
A�����Ӽ� B�����ۼ�
C�������� D����� E�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�������и�һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��10�֣�ÿ��2�֣���ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺

��1��ijѧ����������Ũ���������ˮ����500 mL���ʵ���Ũ��Ϊ0.30 mol/Lϡ���ᡣ
�ٸ�ѧ����Ҫ��ȡ mL����Ũ����������ơ�
������ʱ������ȷ�IJ���˳����(����ĸ��ʾ��ÿ����ĸֻ����һ��) ��
A.��30mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ������ҡ������ƿ
B.����Ͳȷ��ȡ����Ũ���������������ر���ע��ʢ������ˮ��Լ30 mL�����ձ��У��ò���������������ʹ���Ͼ���
C.������ȴ�������ز�����ע��500mL������ƿ��
D.������ƿ�IJ������ǽ����ߵ�ҡ��
E.���ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�������
F.����������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶���1��2cm��
�������ƹ����У����в�����ʹ�����Ƶ�ϡ�������ʵ���Ũ��ƫ�ߵ��� ��
A.����Ͳ��ȡŨ����ʱ���ӹ۲찼Һ��
B.ҡ�Ⱥ�Һ���½�������ˮ
C.����ʱ���ӿ̶���
D.������ǰ����ͬŨ�ȵ�ϡ������ϴ����ƿ
��2���ֽ�200mL0.30mol/L��������50mL0.80mol/LCaCl2��Һ���(��Ϻ�����仯���Բ���)��������Һ��Cl�������ʵ���Ũ���� mol/L��
��3���ڱ�״���£���_____________L HCl��������1000 mLˮ��(ˮ���ܶ�Ϊ1 g/cm3)������������������Ϊ36.5%�����ᡣ(����С�����һλ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010������ʡ������У������߶���ѧ�����п��Ի�ѧ�� ���ͣ�ѡ����
��10�֣�ÿ��2�֣��ڻ�ѧ��Ӧ�У�ֻ�м�����������ƽ�������ߵö�ķ�Ӧ����ӷ�����ײʱ�ſ��ܷ�����ѧ��Ӧ����Щ���ӳ�Ϊ����ӣ�ʹ��ͨ���ӱ�ɻ���������ṩ������ȵ������л�ܣ��䵥λͨ����kJ/mol��ʾ��������۲���ͼ��Ȼ��ش����⡣

��1��ͼ����ʾ��Ӧ��_________������ȡ����ȡ�����Ӧ���÷�Ӧ________�����Ҫ������Ҫ�������ȣ��÷�Ӧ�ġ�H��____________���ú�E1��E2�Ĵ���ʽ��ʾ����
��2����֪�Ȼ�ѧ����ʽ��H2��g����0.5 O2��g����H2O��g������H����241.8 kJ��mol
�÷�Ӧ�Ļ��Ϊ167.2 kJ��mol�������淴Ӧ�Ļ��Ϊ____________________��
��3������ͬһ��Ӧ��ͼ�����ߣ�����ʵ�ߣ�����ȣ���ܴ�ͣ�����ӵİٷ������࣬��Ӧ���ʼӿ죬����Ϊ����ܵ�ԭ����_________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com