

 Һ����B�У������Ŀ����
Һ����B�У������Ŀ����  ��
��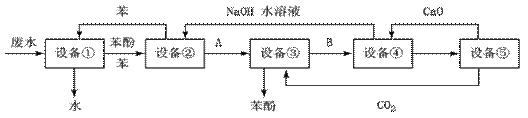
 â���̸������Ծ�ϵ�д�
â���̸������Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
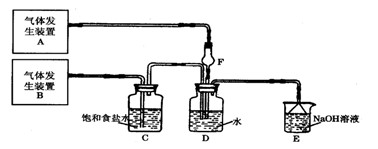
 �� �Ȼ���ϡ��Һ �� �Ȼ�������Һ �� ���軯����Һ
�� �Ȼ���ϡ��Һ �� �Ȼ�������Һ �� ���軯����Һ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A��������ͨ������ԭ����ʯ��ʯ��ʯӢ������ڲ�����¯�з������ӵ�������ѧ�仯 |
| B����ҵ�Ϻϳɰ�û�в��ø����ѹǿ�Ǵ��豸�Ͷ���Ҫ���濼�ǵ� |
| C�����ȷ�ֻ������������ʱӲ�ȵ�Ӳˮ |
| D����ҵ�ϲ��õ�������Ȼ������������� |
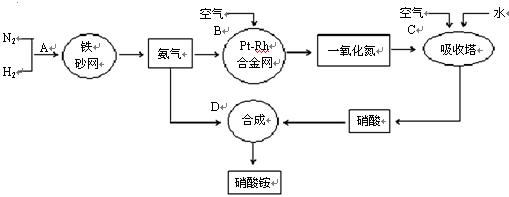
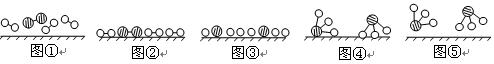
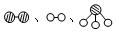 �ֱ��ʾN2��H2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں�ͼ�۵ĺ���ֱ��� �� ��
�ֱ��ʾN2��H2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں�ͼ�۵ĺ���ֱ��� �� �� 7N2+12H2O��NOҲ�����Ƶķ�Ӧ��
7N2+12H2O��NOҲ�����Ƶķ�Ӧ��| | ע������ | ���� |
| �� | | |
| �� | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
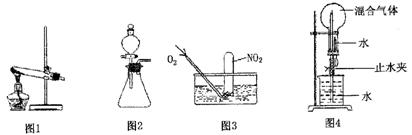
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
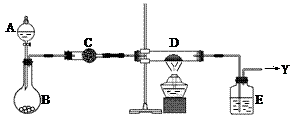
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���������ֳ�ɫ�㷨��ɫ�������Դ����ɫ���ʵķ��� |
| B������������ɫ����û�з������� |
| C���÷۱�Ҳ���Զ�Ҷ����a��Ҷ����b�������ϲ�����ԭ����ֽ�ϲ���һ�� |
| D��������Ҫ��������Ա����������к��ʵ��ܽ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��



| A��ʵ������ȡ��������������ʱ��Ũ��������������ˮ�� |
| B����ͭ˿���Ⱥ����뵽�Ҵ��У���Ӧ��ͭ˿���������� |
| C���ñ���ȡ��ˮ�е��壬��Һʱ�л���ӷ�Һ©�����Ͽڵ��� |
| D������ȡ��������������ʱ�������Թ��м���Ũ���ᣬ�ټ����Ҵ�������Ļ��Һ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com