
�����12�֣�
����������ϩ������Ҫ�Ļ�����Ʒ�����������107t���ҡ�������ʵ�����Ʊ�������ϩ�Ĺ�ҵ�������ж��ֲ�ͬ������
���������գ�
(1)ʵ������ȡ���������������˶������̡�Ũ�����Ũ���ᣬ����Ҫ_________��________����д�Լ�����Һ���ƣ�
(2)ʵ������2.00mol/L�������Ư�۾�[�ɷ�ΪCa(ClO)2��CaCl2]��Ӧ�����������Ȼ��ƺ�ˮ��������2.24L����״����������������Ӧ������Ϊ_________m,l��
(3)ʵ����ͨ���������ſ������ռ����������һ����ʵ�飬��֤���ռ����������Ƿ��п�����
_________________
4)��ҵ���õ�ʯ����Ȳ��������ϩ�ķ�Ӧ���£�
CaO��3C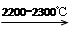 CaC2��CO
CaC2��CO
CaC2��2H2O��HC��CH����Ca(OH)2
HC��CH����HCl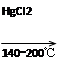 CH2=CHCl
CH2=CHCl
��ʯ����Ȳ�����ŵ������̼���Ʒ���ȸߣ����Ҳ�������ʯ����Դ��
��ʯ����Ȳ����ȱ����___________________��_____________________��
(5)�����������Ӧ���Ƶ�ClCH2CH2Cl��ClCH2CH2Cl���ȷֽ�õ�����ϩ���Ȼ��⡣���һ������ϩ������Ϊԭ����ȡ����ϩ�ķ���������ԭ����ѡ�����û�ѧ����ʽ��ʾ������ע����Ӧ��������
Ҫ�ٷ�Ӧ�������Ȼ��������������ϩ���Ʊ����ڲ��ٲ���������Һ��
___________________________________
(1)�����Ȼ�����Һ������������Һ (2)100
(3)���Թ��ռ��������ռ������Թܵ���������������Һ�У��۲��Թ������������塣
(4)���ܺ� ����Ⱦ����
(5)CH2=CH2��Cl2��ClCH2CH2Cl ClCH2CH2Cl��CH2=CHCl��HCl HC��CH��HCl��CH2=CHCl
�������������(1)Ũ�����ӷ������ɵ������к����Ȼ��⣬Ӧ���ñ����Ȼ�����Һ��ȥ�������ж���Ҫβ��������������������Һ���ա�
(2)���ݷ�Ӧ����������֪����Ӧ�Ļ�ѧ����ʽΪ4HCl��Ca(ClO)2��CaCl2��2H2O��2Cl2������Ӧ���������������ʵ�����0.1mol���������Ȼ�������ʵ�����0.2mol�������Ũ����2.00mol/L�������Ҫ�������Һ�������100ml��
(3)������������������Һ��ȫ��Ӧ�����������������Ʋ���Ӧ���ݴ˼��飬���Թ��ռ��������ռ������Թܵ���������������Һ�У��۲��Թ������������塣
(4)�������̿�֪����Ӧ��Ҫ���£����ȱ��֮һ�Ǹ��ܺģ���Ҫ�Ȼ����������������ؽ��������ȱ��֮���ǻ���Ⱦ������
(5)�����������Ӧ���Ƶ�ClCH2CH2Cl��ClCH2CH2Cl���ȷֽ�õ�����ϩ���Ȼ��⣬�������Ȼ����������Ȳ��Ӧ����������ϩ����˷���ΪCH2=CH2��Cl2��ClCH2CH2Cl��ClCH2CH2Cl��CH2=CHCl��HCl��HC��CH��HCl��CH2=CHCl��
���㣺���������Ʊ������������㡢�����Ʊ���������Լ����۵�


 ��һ������Ԫͬ�����ؾ�ϵ�д�
��һ������Ԫͬ�����ؾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ƾ���[CaO2��8H2O]���ȶ����ʰ�ɫ������ˮ��������������Һ���㷺Ӧ���ڻ���ɱ��������������
��.�������ƾ�����Ʊ���
��ҵ������CaO2��8H2O����Ҫ�������£�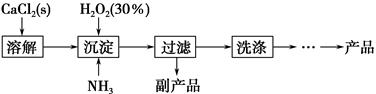
��1��������������ȡCaO2��8H2O�Ļ�ѧ����ʽ��_________________________________��
��2������ʱ���ñ�ˮ�����¶���10 �����º�ͨ�������NH3�������ԭ��ֱ��Ǣ�__________________________����_____________________________��
��.�������ƾ��庬���IJⶨ��
ȷ��ȡ0.300 0 g��Ʒ����ƿ�У�����30 mL����ˮ��10 mL 2.000 mol��L��1 H2SO4����0.020 0 mol��L��1 KMnO4����Һ�ζ����յ㡣�ظ������������Ρ�H2O2��KMnO4��Ӧ�����ӷ���ʽΪ2MnO4-��5H2O2��6H��=2Mn2����5O2����8H2O
�ζ��յ�۲쵽������Ϊ_______________________________________��
��4�����ݱ������ݣ������Ʒ��CaO2��8H2O������������д��������̣���
KMnO4����Һ�ζ�����
| �ζ����� | ��Ʒ������/g | KMnO4��Һ�����/mL | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 0.300 0 | 1.02 | 24.04 |
| 2 | 0.300 0 | 2.00 | 25.03 |
| 3 | 0.300 0 | 0.20 | 23.24 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
���������(K2S2O8)��һ����ɫ�ᾧ���������Ҵ�����ǿ�����ԣ��ֽ⡣ʵ�����Ʊ���������ؿ�ͨ�����µ��KHSO4����Һ�õ���
ʵ�鲽�����£�
����1����ȡ40gKHSO4�ܽ�90mL����ˮ��������Թܣ��Թܽ��ڱ�ˮԡ��(װ�ü�ͼ9)������ȴ��5�����¡�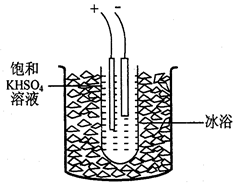
����2���2h��ÿ��Լ��Сʱ��һ�α�
����3���������ռ���©���У�ֱ�����Ҵ�������ϴ�Ӻ���
����4���������
����5���������Ѻ��Ҵ�
��1������ܷ�Ӧ�Ļ�ѧ����ʽΪ ��
��2���������У���������������ʹʪ���KI-���۱�������ɫ�������壬�����������(�ѧʽ)��
��3������2ÿ����СʱҪ����ձ����ӱ��飬��ԭ���� ��
��4������5�����Ҵ�������ʱ���õIJ��������� ��
��5��ȡ�õ�����Ʒ0.2500g����30mLˮ����4gKI����סƿ��������ֹ15min������1mL�����ᣬ����cmol��L- 1Na2S2O3��Һ�ζ���(S2O82- +3I- =2SO42- +I3-��I3- I2+I-��2S2O32-+I2=2I- + S4O62-)
I2+I-��2S2O32-+I2=2I- + S4O62-)
���ܽ�ʱ������KI������סƿ������Ŀ���� ��
�ڱ�ʵ�����õ�ָʾ��Ϊ ��
�������εζ�����Na2SO3��ҺVmL���ɱ��ν�����㣬��Ʒ��K2S2O8�Ĵ���Ϊ(�ú�c��V�Ĵ���ʽ��ʾ)��
��6��������ѧ�ϼ���Mn2+��Ag+����K2S2O8��Һ��Mn2+����Ϊ��ɫ��MnO4-���÷�Ӧ�����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������16�֣���.ʵ���Ҿ���Ҫ��ȡij�����岢��֤����������ʡ�
��1������װ���ʺ���β�����յ��� ������ţ���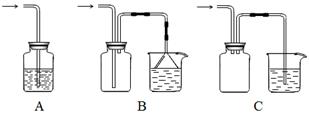
��2��������װ����ͨ������X��A��Ʒ����Һ��ɫ����X������___________�����������֣����Ҫ֤��X��SO2����,�������IJ����ǣ�________________________________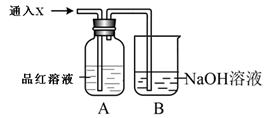
��.ʵ������FeSO4��Һ��NaOH��Һ��Ӧ��ȡFe(OH)2��ȴ���ѿ����ȶ��İ�ɫ��������ͬѧ�����һ���µ�ʵ�鷽����������������ɰ�ɫ��������ɫ����ת���ɺ��ɫ������������������¿հף�
��1��ȡһ�������0.1mol.L-1NaOH��Һ�����ձ��У�_________���ٵ��뼸��ֲ���ͣ�
��2��ѡ��װ��___________����ס����ҡ���������1��������Һ���뵽װ���в���ͼʾ��װ��װ�á�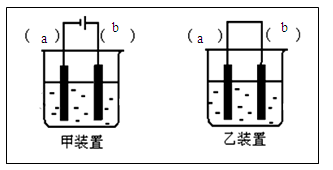
��3������ѡ���װ���ϱ����缫���Ϸֱ�Ϊ____________��________________
��4��ʵ���п���___________�����ȶ��İ�ɫFe(OH)2������
��5�����Ҫ������ɫ����ת��Ϊ���ɫ�����ĵ����������IJ�����______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����ɫ������A������ɫ���ߵ�ѹ���������ص㣬�����������˵绯ѧ��ĸ߶����ӡ��ڳ��º���������£�������A�����ȶ��Ĵ��ڣ�������ˮ��Һ�в��ȶ���һ��ʱ���ת��Ϊ���ɫ������ͬʱ����һ�����嵥�ʡ�ij��ȤС���ͬѧ�Ի�����A������ɷ�����ȷ��A�н�����O��K��Fe����Ԫ�ء�ȡ3.96g������A�ķ�ĩ����ˮ���μ�������ϡ���ᣬ��Ӧ�����Һ�м��뺬��0.08mol KOH����Һ��ǡ����ȫ��Ӧ�����ˣ���ϴ�Ӻ�ij���������գ��õ�����ɫ�����ĩ1.60g����������Һ��һ�������������ɵõ�һ�ִ����IJ����ᾧˮ����10.44g��
��1��������A�Ļ�ѧʽΪ ��������A��H2O��Ӧ�����ӷ���ʽΪ ��
��2��������A������Ϊһ�֡���ɫ��Ч��ܡ�ˮ��������ԭ���� ��
��3��������A���Ʊ�����ͨ������������д����KOH�����������ô�������������������Ʊ�A�Ļ�ѧ����ʽ ��
��4��Ŀǰ��������Ի�����A���ȶ��Խ����˴�����̽������ȡ����һ���Ľ�չ�������������п����������Aˮ��Һ�ȶ��Ե���
| A���������� | B��KOH | C������ | D��Fe(NO3)3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�����12�֣�
�ҹ������������[(NH4)3PO4]�ŷŵķ�����ʯ��ȡ���Ტ����ˮ��ļ����о���óɹ�����֪��ʯ����Ҫ�ɷ���Ca3(PO4)2��������������������£�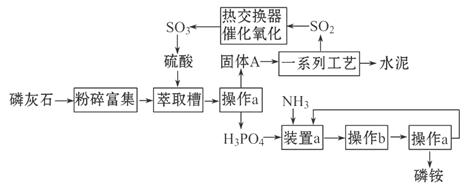
38������a��������___________��ʵ�����н��д˲����ķDz���������Ʒ��___________����ʵ�����в���b��������______________________��
39��װ��a������������ʽ�Σ����ǵĻ�ѧʽ�ֱ���_______________________��
40������A��һ�����е����ʣ��仯ѧʽ��_________________��
41���Ƚ�������ʵ�����Ƚ�����װ�á���ѧʵ����Ҳ���������Ƚ�����ʵ��ij��ʵ��Ŀ�ģ�����Һ�Ƚ���ʱͨ��ʹ�õ�������________________________��
42. ��������������β�����˺���N2��O2�⣬������SO2������SO3�������������ڲⶨ����β����SO2�������Լ���__________��
a��NaOH��Һ����̪��Һ b��KMnO4��Һ��ϡ����
c. ��ˮ��������Һ d����ˮ����̪��Һ
���õIJ�������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ȤС��Ϊ̽�����Ļ���������ʣ��������ͼ��ʾʵ��װ�ã�AΪ���巢��װ�á�
����ͼ���Ӻø����������װ�������Ժ��Ƚ�C����˿�����������ȣ��ٽ�A����������ɫ����ͨ������װ�á�Ƭ�̺�ɹ۲쵽F��ͭƬ�����ܽ⡣��ش��������⣺
(1)A�Ʊ���A�������Լ��������й���������ѡȡ����NH4HCO3����NH4Cl����Ca(OH)2)���Ӧ��װ�ÿ�����________(����ĸ)��
(2)д�����װ��C�з�����Ӧ�Ļ�ѧ����ʽ________��C������Ƭ�̺�ȥ�ƾ��ƣ���˿�Ա��ֺ��ȣ�ԭ����________________________________________��
(3)ͭƬ��ȫ�ܽ����Fװ������Һ����ɫ����ˮϡ�ͺ����ɫ����ͬѧ�ó����ֽ��ۣ���ŨCu(NO3)2��Һ����ɫ��ϡCu(NO3)2��Һ����ɫ����Cu(NO3)2��Һ����ɫ������ɫ��������Һ�ܽ������NO2�������ʵ����֤��һ�ֽ�����ȷ______________________________________________________________��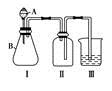 (4)����ȤС��ͬѧ��ͬ�������ͼ��ʾ��ʵ��װ�ã�����װ�â���ȡ���壬��ش��������⣺
(4)����ȤС��ͬѧ��ͬ�������ͼ��ʾ��ʵ��װ�ã�����װ�â���ȡ���壬��ش��������⣺
�ټ�ͬѧ��Ϊ������װ�â�����ռ�H2��NH3�����壬�������ռ�O2��NO��������______________________________________________��
����ͬѧ��Ϊ������װ�â������Ľ�(���ı�����װ��)�����ռ�NO��O2���Ľ��ķ�����__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
(12 ��)����ʯ��������Ṥҵ��β��(��NO��NO2)��Ӧ�����ܾ���β�������ܻ��Ӧ�ù㷺��Ca(NO2)2���䲿�ֹ����������£�
(1)һ�������£�NO ��NO2�������з�Ӧ��NO(g)+NO2(g) N2O3(g)����ƽ�ⳣ������ʽΪK = ��
N2O3(g)����ƽ�ⳣ������ʽΪK = ��
(2)���������в�����-Һ�����Ӵ�����(β�������������룬ʯ�����������������)�� ��Ŀ���� ;������ѭ��ʹ�ã���������Ҫ�ɷ��� (�ѧʽ)��
(3)�ù��������NO ��NO2���ʵ���֮�Ƚӽ�1 ��1����n(NO)��n(NO2)>1 ��1����ᵼ�� ;��n(NO)��n(NO2)<1 ��1����ᵼ�� ��
(4)��������Һ�豣�������ԣ���������Һ��Ca(NO2)2�ᷢ���ֽ⣬����֮һ��NO���䷴Ӧ�����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����̼��ƹ㷺Ӧ���������ϡ���ֽ����ѧ���ġ���ī��Ϳ�ϡ��ܷ⽺�뽺ճ������ҵ����ŨCaCl2��Һ��ͨ��NH3��CO2�������Ƶ�����̼��ơ�ijУѧ��ʵ��С�������ͼ��ʾװ�ã���ȡ�ò�Ʒ��D��װ��պϡ�������֬�ޣ�ͼ�мг�װ������ȥ��
��ѡ�õ�ҩƷ�У�
a��ʯ��ʯ��b�������Ȼ�����Һ��c��6 mol/L���d���Ȼ�泥�e����������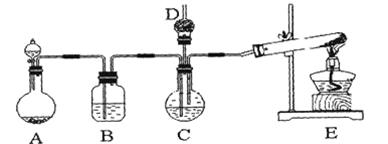
��1��A���Ʊ�����ʱ������ҩƷ�ǣ�ѡ����ĸ��ţ� ��
��2��B��ʢ�б���̼��������Һ���������� ��
��3��д����ȡ�����Ļ�ѧ����ʽ ��
��4����ʵ������У���C��ͨ�����������Ⱥ�˳��ģ�Ӧ��ͨ������Ļ�ѧʽ ��
��5������D���ڴ��Ƿ��а����ݳ��ķ����� ��
��6��д��������̼��ƵĻ�ѧ����ʽ ��
��7����ʵ��������а����ݳ���Ӧѡ������ װ�û��գ�����ţ���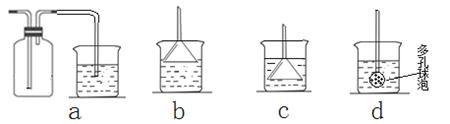
�������������Ȼ����Ʒ�к�������̼�����ơ�Ϊ�˲ⶨ�Ȼ�淋�������������ѧ��ʵ��С�������������ʵ�����̣�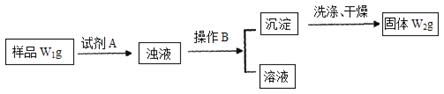
�Իش�
��1�������Լ�A�Ļ�ѧʽΪ
��2��B����������
��3����Ʒ���Ȼ�淋���������Ϊ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com