���𰸡�
��������1��������Һϡ��ǰ���������ʵ��������������Ũ����������
��2������ʵ������IJ����Լ�ÿ��������Ҫ����ȷ����Ӧ����������
��3������ƿ��ƿ���Ĵ֣��ή������ƿ�ľ�ȷ�ȣ�
��4���������������ʵ����ʵ��������Һ�������Ӱ�죬����c=
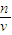
�����жϣ�
��5�����ݷ���ʽ����μӷ�Ӧ��п����������ʵ���������m=nM����п��������
��Һ��n��H
+��=n��H
2SO
4�����������������ʵ���Ũ�ȱ仯������������Ũ�ȱ仯���������ӿ�ʼŨ�ȼ�ȥ������Ũ�ȱ仯�����ڷ�Ӧ����Һ�е������ӵ����ʵ���Ũ�ȣ�
����⣺��1������ϡ�Ͷ��ɣ�ϡ��ǰ�����ʵ����ʵ������䣬������ŨŨ������������Ũ��������ΪxmL��
��xmL×18mol/L=200mL×0.9mol/L����ã�x=10.0������Ӧ��ȡ��Ũ���������10.0mL��
�ʴ�Ϊ��10.0��
��2�����������м��㡢��ȡ��ϡ�͡���Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ���������Ͳ��ȡ���õ���ͷ�ιܣ�Ũ���ᣬ���ձ���ϡ�ͣ��ò��������裬��ȴ�����º�ת�Ƶ�200mL����ƿ�У����ò�����������ϴ��2-3�Σ���ϴ��Һת�Ƶ�����ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμ�����Һ��Һ����̶���ˮƽ���У��Ǻ�ƿ���ߵ�ҡ�ȣ�
������Ҫ������Ϊ�����������ձ�����ͷ�ιܡ���Ͳ��200mL����ƿ��
�ʴ�Ϊ����ͷ�ιܣ���������
��3������ƿ��ƿ���Ĵ֣��ή������ƿ�ľ�ȷ�ȣ�
��ѡ��B��
��4��������ƿδ���T����������Һ�������Ҫ���ݣ�����ƿ�����������������ˮ������ҺŨ����Ӱ�죻
������ʱ���ӿ̶��ߣ�������Һ���ƫС��������ҺŨ��ƫ��
�ʴ�Ϊ�����ڣ����ڣ�
��5��Zn+H
2SO
4=ZnSO
4+H
2��
1mol 1mol 22.4L
x y 0.448L
����x=
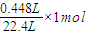
=0.02mol��
y=x=0.02mol��
���Բμӷ�Ӧп������Ϊ0.02mol×65g/mol=1.3g��
������Ũ�ȱ仯��Ϊ��c��H
+��=
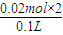
=0.4mol/L��
���Է�Ӧ����Һ�е������ӵ����ʵ���Ũ��Ϊ2×0.9mol/L-0.4mol/L=1.4mol/L��
�ʴ�Ϊ��1.3g��1.4mol/L��
������������Ҫ������һ�����ʵ���Ũ����Һ�����ƣ�ע���c=
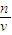
��������ԭ����ע��Ũ�����ϡ�Ͳ�����

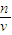 �����жϣ�
�����жϣ�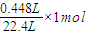 =0.02mol��
=0.02mol��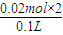 =0.4mol/L��
=0.4mol/L��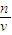 ��������ԭ����ע��Ũ�����ϡ�Ͳ�����
��������ԭ����ע��Ũ�����ϡ�Ͳ����� 

