
| A����ԭ�ӵ��ӻ����ͷ����˸ı� | B��������״�����˸ı� |
| C�����Ļ�ѧ���ʷ����˸ı� | D�����еļ��Ƿ����˸ı� |

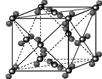


 ��֪��1molˮ�к���2mol����������ȣ����»���+��������ڱ�����������51kJ/mol��ˮ���Ӽ�ķ��»�����11kJ/mol�����Ա�����������ġ����ܡ���(51kJ/mol��11kJ/mol)��2��20kJ/mol��
��֪��1molˮ�к���2mol����������ȣ����»���+��������ڱ�����������51kJ/mol��ˮ���Ӽ�ķ��»�����11kJ/mol�����Ա�����������ġ����ܡ���(51kJ/mol��11kJ/mol)��2��20kJ/mol�� �����ɴ�������ӵ����ӷ���ʽCu2++4H2O��[Cu(H2O)4]2+��
�����ɴ�������ӵ����ӷ���ʽCu2++4H2O��[Cu(H2O)4]2+��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ������/kJ��mol-1 | I1 | I2 | I3 | I4 |
| A | 578 | 1 817 | 2 745 | 11 578 |
| B | 738 | 1 451 | 7 733 | 10 540 |
| ���ۼ� | C��C | C��N | C��S |
| ����/kJ��mol-1 | 347 | 305 | 259 |
| ���Ӿ��� | NaCl | KCl | CaO |
| ������/kJ��mol-1 | 786 | 715 | 3 401 |


�鿴�𰸺ͽ���>>
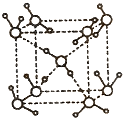
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ɶ�����̼�ϳɽ��ʯ�ǻ�ѧ�仯 | B�����ʯ��̼��һ��ͬλ�� |
| C���Ʊ�������������̼���� | D�����ʯ��ֻ���зǼ��Թ��ۼ� |
�鿴�𰸺ͽ���>>
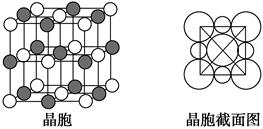
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 �ڹ�����ˮ����ת��Ϊ
�ڹ�����ˮ����ת��Ϊ ��д��
��д�� �ļ۲�����Ų�ͼ____��
�ļ۲�����Ų�ͼ____�� ��
�� ����λ��Ϊ____��NH3���ӵ�����ԭ���ӻ���ʽΪ____��
����λ��Ϊ____��NH3���ӵ�����ԭ���ӻ���ʽΪ____�� �ȡ�CO��N2���ڵȵ����壬��CO������
�ȡ�CO��N2���ڵȵ����壬��CO������ ����
���� ����Ŀ��Ϊ____��д����CO��Ϊ�ȵ������һ�������ӵ����ӷ���____��
����Ŀ��Ϊ____��д����CO��Ϊ�ȵ������һ�������ӵ����ӷ���____�� O���þ�����Ni3����Ni2����������֮��Ϊ____��
O���þ�����Ni3����Ni2����������֮��Ϊ____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com