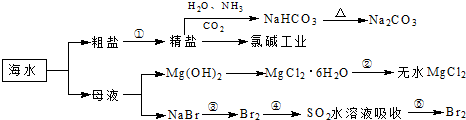
�������зḻ��ʳƷ���������Դ��ҩ���ˮ����Դ����ͼΪ��ˮ���õIJ��ֹ��̣�
�ش������й����⣮
��1�������й�˵����ȷ����
��
A��Ŀǰ��������Ҫʹ�����ġ���ˮ�������������øߴ��ȵ�ˮ
B���ó����ʯ��ˮ�ɼ���NaHCO
3��Na
2CO
3C���ڵڢۡ��ܡ��ݲ����У���Ԫ�ؾ�������
D����ҵ��ͨ����ⱥ��NaCl��Һ��ȡ������
��2��д���ڢ۲���Ļ�ѧ��Ӧ����ʽ
��
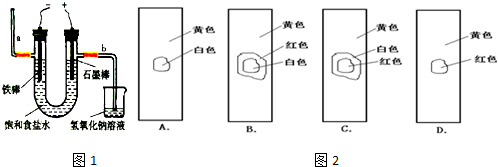
��3����ҵ�����õ�ⱥ��ʳ��ˮ���Ƶ���Ҫ������Ʒ���ֳ�Ϊ���ȼҵ���� �����Ĺ�ҵ��ȡװ����ͼ1���ش�
��������ʳ��ˮ�к��з�̪��ͨ���
����a��b�����ȱ�죮
�ڵ�ⷴӦ�Ļ�ѧ����ʽΪ
��
�������Ƴ���ˮ���ò�����պȡ������ˮ����pH��ֽ�в����۲쵽��������
��
��4����ҵ����NaCl��NH
3��CO
2��Ϊԭ�����Ƶ�NaHCO
3����������������йط�Ӧ�Ļ�ѧ����ʽΪ��
NH
3+CO
2+H
2O�TNH
4HCO
3��
NH
4HCO
3+NaCl�TNaHCO
3��+NH
4Cl��
2NaHCO
3Na
2CO
3+CO
2��+H
2O
ij�С����������Ƽ�ԭ��������̼�����Ƶ��Ʊ�ʵ�飮
��һλͬѧ��������̼����ͨ�뺬���ı���ʳ��ˮ���Ʊ�̼�����ƣ�ʵ��װ����ͼ3��ʾ��ͼ�мг֡��̶��õ�����δ���������Իش������й����⣺
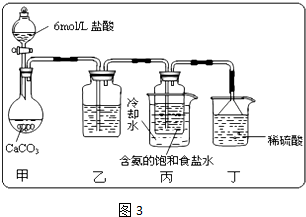
����װ���е��Լ���
����������
��
��װ����ϡ�����������
��
��ʵ����������NaHCO
3 ����IJ�����
���������������ƣ����ò�������Ҫ�IJ���������
��
��̼�������������ù���12.28g��������ʯ��ˮ��ַ�Ӧ�����ó�����ϴ�ӡ���������Ϊ12.00g�������ù�����̼���Ƶ���������Ϊ
��
����������Ƴ�һ��ʵ������ȡ����̼�����Ƶķ�����
��