

| Al2O3 | Fe2O3 | |
| ������ | 55% | 16% |
| ���� | 15% | 48% |
| ||
| 108��10.20t |
| 204 |
| 0.16t |
| 0.48 |
| 1 |
| 3 |
| 1t��102 |
| 54 |
| 1 |
| 3 |
| 1.89t |
| 0.50 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ԭ�Ӱ뾶��C��D��A��B |
| B��ԭ��������C��D��B��A |
| C�����Ӱ뾶��D��C��A��B |
| D�����ʵĻ�ԭ�ԣ�B��A��C��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Na+��Fe2+��SO42-��K+ |
| B��HCO3-��K+��NO3-��SO42- |
| C��SO42-��Cl-��CO32-��Na+ |
| D��NH4+��Cl-��Na+��Ba2+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ʯ������ˮ |
| B����̿��ˮ�����ķ�Ӧ |
| C��þ��ϡ���ᷴӦ |
| D�������������Ȼ�茶����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
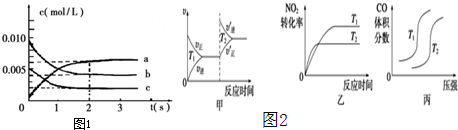 ���Ǵ����к����������壬�о������仯�������������Ҫ�����壮
���Ǵ����к����������壬�о������仯�������������Ҫ�����壮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �ݲⶨ��ָʾ����̪�Ľṹ��ʽ�ɱ�ʾΪ��
�ݲⶨ��ָʾ����̪�Ľṹ��ʽ�ɱ�ʾΪ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��1��P4�����ף�s��+5O2��g���TP4O10��s����H=-2983.2kJ/mol
��1��P4�����ף�s��+5O2��g���TP4O10��s����H=-2983.2kJ/mol| 5 |
| 4 |
| 1 |
| 4 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com