
(8��)��1��A��B��Ԫ�ؾ�λ�����ڱ��е������ڣ����ǵ�ԭ�Ӻ�������������֮��Ϊ7������AԪ�ص�ԭ�Ӻ�������������Ϊ1����������γɻ�����A2B���������������ش�
��д��A��B��Ԫ�ط��ţ�A ��B
��д��������A2B�ĵ���ʽ ��
(2) ����A��B��C��D�������ʣ�����֮���ܷ�������ͼ��ʾ��ת����ϵ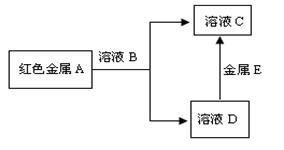
��ҺB��C�ж����н���Ԫ��E����ҺB����ɫΪ��ɫ�������������Ϣ�ش��������⣺
��д������EԪ�ط��ţ�
��д������A����ҺB��Ӧ�����ӷ���ʽ��
��Ϊ�˼�����ҺB�н��������ӣ�ͨ��������Լ���
��1���� A�� Na B�� S ��
��2���� Fe ��2Fe3+ + Cu = 2Fe2++Cu2 + ��KSCN��Һ
���������������1��A��B��Ԫ�ؾ�λ�����ڱ��е������ڣ����ǵ�ԭ�Ӻ�������������֮��Ϊ7������AԪ�ص�ԭ�Ӻ�������������Ϊ1������A��Na��BԪ�ص�����������Ϊ6����B��S��
��2��A�Ǻ�ɫ����������A�ǽ���Cu����ҺB���н���EԪ�أ�����Һ�ǻ�ɫ��˵��B��Һ�Ǻ�Fe3+������Һ������A��B��ӦΪ2Fe3+ + Cu = 2Fe2++Cu2 + ������E��Fe������D����Fe��Ӧ����D�Ǻ���Cu2+������Һ��C�Ǻ���Fe2+������Һ������Fe3+���ӳ���KSNC��Һ�����Ƿ���Ѫ��ɫ������
���㣺��ѧ����ʽ��д
��������ѧ�ƶ�����һ���ۺ��Խ�ǿ�����⣬��Ԫ�ؼ����������ʺ�������������������ѧ�����֪ʶ,��������ѧ�Ƽ��ۺϡ��������ɿ���ѧ���Ի�ѧ֪ʶ������̶ȣ�����Ҫ��������ѧ�����ۺϷ���������˼ά���������ͼ��ķ���:��ؼ�����Ѱ��"ͻ�ƿ�"��"ͻ�ƿ�"����ץ"��"�֣�����������ɫ������״̬��������ζ�����ⷴӦ���������������Ʒ���������;�ȡ�


 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ||
| ||
|
| 1 |
| 2 |
|
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��
�� ����д���֣�
����д���֣� ��
�� ����д���֣�
����д���֣�



| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(8��)��ͼ�е缫a��bΪFe�����AgƬ���缫c��d����ʯī�缫��ͨ��һ��ʱ�����c��d�����Ϲ��ռ���336ml(��״��)���塣�ش�
(1)ֱ����Դ�У�MΪ_________________����
(2)����Fe����϶���һ����Ag��
��Fe���ӦΪ_________����(��a��b)
��Fe�������_________g��
(3)X��ҺΪ____________����Ũ��____________��(�������С�����䡱)
(4)��NaOH��Һ������������5.00%��Ϊ5.02%����ʽ����ԭNaOH��Һ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ�ϲ����и߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
(8��)��ͼ�е缫a��bΪFe�����AgƬ���缫c��d����ʯī�缫��ͨ��һ��ʱ�����c��d�����Ϲ��ռ���336ml(��״��)���塣�ش�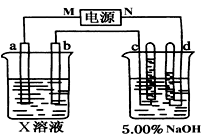
(1)ֱ����Դ�У�MΪ_________________����
(2)����Fe����϶���һ����Ag��
��Fe���ӦΪ_________����(��a��b)
��Fe�������_________g��
(3)X��ҺΪ____________����Ũ��____________��(�������С�����䡱)
(4)��NaOH��Һ������������5.00%��Ϊ5.02%����ʽ����ԭNaOH��Һ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
(8��)��ͼ�е缫a��bΪFe�����AgƬ���缫c��d����ʯī�缫��ͨ��һ��ʱ�����c��d�����Ϲ��ռ���336ml(��״��)���塣�ش�
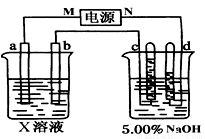
(1)ֱ����Դ�У�MΪ_________________����
(2)����Fe����϶���һ����Ag��
��Fe���ӦΪ_________����(��a��b)
��Fe�������_________g��
(3)X��ҺΪ____________����Ũ��____________��(�������С�����䡱)
(4)��NaOH��Һ������������5.00%��Ϊ5.02%����ʽ����ԭNaOH��Һ��������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com