
����Ŀ����һ��������Mg-Al�Ͻ�Ͷ��100mLһ�����ʵ���Ũ�ȵ�ijHCl��Һ�У���ַ�Ӧ����Ӧ�����Һ����μ���һ�����ʵ���Ũ�ȵ�NaOH��Һ�����ɳ���������������NaOH��Һ�������ϵ����ͼ���ش��������⣺

��1��д��OA�κ�BC�η�Ӧ�����ӷ���ʽ��
OA��___________ �� BC��_____________��
��2��ԭMg-Al�Ͻ��������_____________��
��3��ԭHCl��Һ�����ʵ���Ũ����________________��
��4������NaOH��Һ�����ʵ���Ũ����____________��
���𰸡�H++OH-=H2O�� Al(OH)3+OH-=AlO2-+2H2O 5.1g 6mol/L 5mol/L
��������
������Ҫ���������仯���
Mg-Al�Ͻ���HCl��Һ��Ӧ������MgCl2��AlCl3��MgCl2��NaOH��Ӧ����Mg(OH)2������AlCl3��NaOH��Ӧ����Al(OH)3������NaOH����ʱ��Al(OH)3������NaOH��Һ��Ӧ����NaAlO2��Һ����ͼ��֪����ʼ����NaOH��Һʱ������������˵��Mg-Al�Ͻ���HCl��Һ��Ӧ��HCl��ʣ�࣬��OA�η���NaOH��HCl������кͷ�Ӧ��AB�η���������Ӧ����Mg(OH)2��Al(OH)3������BC�γ������������٣�����Al(OH)3�����ܽⷴӦ����NaAlO2�����ճ���ΪMg(OH)2��
��1�����ݷ�����֪OA�ε����ӷ���ʽΪ��H++OH-=H2O��BC�ε����ӷ���ʽΪAl(OH)3+OH-=AlO2-+2H2O��
��2�����ݷ���C����ڳ���ΪMg(OH)2������Ϊ5.8g������þԪ���غ㣬�� Mg�����ʵ���Ϊn(Mg)=n[Mg(OH)2]=0.1mol������B�����������Ϊ13.6g��C���������Ϊ5.8g����B�����ɵ�Al(OH)3����������Ϊ7.8g��������Ԫ���غ㣬��Al�����ʵ���Ϊn(Al)=n[Al(OH)3]=0.1mol����ԭMg-Al�Ͻ������Ϊ0.1mol��24g/mol+0.1mol��27g/mol=5.1g��
��3��BC�η�����ӦAl(OH)3+OH-=AlO2-+2H2O��0.1mol Al(OH)3����NaOH�����ʵ���Ϊ0.1mol����Һ���Ϊ20mL����NaOH��Һ�����ʵ���Ũ��Ϊ0.1mol/0.02L=5mol/L��B������NaOH�����ʵ���Ϊ0.12L��5mol/L=0.6mol��B����Һ������ΪNaCl��������Ԫ���غ㣬��ԭ�����к���HCl�����ʵ���Ϊ0.6mol����ԭHCl��Һ�����ʵ���Ũ��Ϊ0.6mol/0.1L=6mol/L��
��4�����ݣ�3���з�����֪NaOH��Һ�����ʵ���Ũ��Ϊ5mol/L���ݴ˽��
Mg-Al�Ͻ���HCl��Һ��Ӧ������MgCl2��AlCl3��MgCl2��NaOH��Ӧ����Mg(OH)2������AlCl3��NaOH��Ӧ����Al(OH)3������NaOH����ʱ��Al(OH)3������NaOH��Һ��Ӧ����NaAlO2��Һ����ͼ��֪����ʼ����NaOH��Һʱ������������˵��Mg-Al�Ͻ���HCl��Һ��Ӧ��HCl��ʣ�࣬��OA�η���NaOH��HCl������кͷ�Ӧ��AB�η���������Ӧ����Mg(OH)2��Al(OH)3������BC�γ������������٣�����Al(OH)3�����ܽⷴӦ����NaAlO2�����ճ���ΪMg(OH)2��
��1�����ݷ�����֪OA�ε����ӷ���ʽΪ��H++OH-=H2O��BC�ε����ӷ���ʽΪAl(OH)3+OH-=AlO2-+2H2O����С���Ϊ��H++OH-=H2O��Al(OH)3+OH-=AlO2-+2H2O��
��2�����ݷ���C����ڳ���ΪMg(OH)2������Ϊ5.8g������þԪ���غ㣬�� Mg�����ʵ���Ϊn(Mg)=n[Mg(OH)2]=0.1mol������B�����������Ϊ13.6g��C���������Ϊ5.8g����B�����ɵ�Al(OH)3����������Ϊ7.8g��������Ԫ���غ㣬��Al�����ʵ���Ϊn(Al)=n[Al(OH)3]=0.1mol����ԭMg-Al�Ͻ������Ϊ0.1mol��24g/mol+0.1mol��27g/mol=5.1g����С���Ϊ��5.1g��
��3��BC�η�����ӦAl(OH)3+OH-=AlO2-+2H2O��0.1mol Al(OH)3����NaOH�����ʵ���Ϊ0.1mol����Һ���Ϊ20mL����NaOH��Һ�����ʵ���Ũ��Ϊ0.1mol/0.02L=5mol/L��B������NaOH�����ʵ���Ϊ0.12L��5mol/L=0.6mol��B����Һ������ΪNaCl��������Ԫ���غ㣬��ԭ�����к���HCl�����ʵ���Ϊ0.6mol����ԭHCl��Һ�����ʵ���Ũ��Ϊ0.6mol/0.1L=6mol/L����С���Ϊ��6mol/L��
��4�����ݣ�3���з�����֪NaOH��Һ�����ʵ���Ũ��Ϊ5mol/L��


 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������з����˷ܼ�����ʧ��ƽ��Ҳ�ܻ����������¡�ij�˷ܼ��Ľṹ��ʽ����ͼ��ʾ�������й�˵����ȷ���� ( ��)
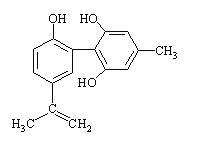
A. �������뱽������ͬϵ���FeCl3��Һ����ɫ
B. ��������KMnO4��Һ����ɫ��ȥ����֤����ṹ�п϶�����̼̼˫��
C. 1 mol�����ʷֱ���Ũ��ˮ��H2��Ӧʱ���������Br2��H2�ֱ�Ϊ2 mol��7 mol
D. �÷����е�����̼ԭ�ӿ��Թ�ƽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ�������£���ˮ��Һ��1 mol Cl����ClOx��(x��1��2��3��4)������(kJ)��Դ�С��ͼ��ʾ�������й�˵����ȷ����
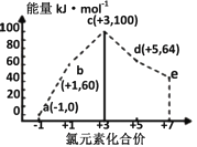
A. a��b��c��d��e�У�c���ȶ�
B. b��a��c��Ӧ�Ļ��Ϊ��Ӧ������������������
C. b��a��d��Ӧ���Ȼ�ѧ����ʽΪ��3ClO��(aq)��ClO3��(aq)��2Cl��(aq)��H����116 kJ��mol��1
D. һ���¶��£�Cl2��NaOH��Һ��Ӧ���ɵIJ�����a��b��d����Һ��a��b��d��Ũ��֮�ȿ���Ϊ11��1��2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й������ʻ����Ӽ����������ȷ����
A. ����ͨ����ˮ����ͭ��ĩ����ĩ������֤��ԭ�����к���ˮ����
B. ����Һ�м�KSCN��Һ����Һ�Ժ�ɫ��֤��ԭ��Һ����Fe3������Fe2��
C. ���հ�ɫ��ĩ������ʻ�ɫ��֤��ԭ��ĩ����Na������K��
D. ��ij��ɫ��Һ�м������ᣬ������ɫ��ζ���壬������ͨ�����ʯ��ˮ����Һ����ǣ�֤��ԭ��Һһ������![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ��������������ʣ����ʵ����֤ȡ����Ӧ����ȥ��Ӧ�IJ������һ�����̽����
�������Թ��м���5mL1mol/LNaOH��Һ��5mL�����飬��Ȼ���Թ���ͼ�̶���ˮԡ���ȡ�

��1��д����ˮԡ���ȵ�ԭ����___________��
��2���Թܿڰ�װһ�����ܵ�����___________��
��3��д����Ӧ�Ļ�ѧ����ʽ________________________________��
��4����ͬѧΪ����ˮ������е������ӣ�ȡ��ȴ��Ļ��Һ��������һС�Թ��У��μ���������Һ���۲��Ƿ�����dz��ɫ��������ͬѧʵ�������Ե�һ��������___________��
����ͼ��ʾ�����Թ��������顢NaOH���Ҵ������Һ����һ��ʱ����ָ������������Һ��ɫ��

��5��д���Թ�A�з�Ӧ�Ļ�ѧ����ʽ_______________________________��
��6����ͬѧ��Ϊ�Թ�B�еĸ������������Һ��ɫ����˵��������������ϩ������˵������?___________(��д����ȷ������������),��������˵�����ɺĽ�����(�����ȷ�����ʲ�����)___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijȩ�Ľṹ��ʽΪ(CH3)2C===CHCH2CH2CHO��ͨ��ʵ�鷽���������еĹ����š�
��1��ʵ������У�Ӧ�ȼ������ֹ����ţ�________��ԭ����______________________��
��2�����������ȩ���ķ�����_____________________________________________����ѧ����ʽΪ____________________________________________________________��
��3�����������̼̼˫���ķ�����__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ��������ܴﵽĿ���ǣ�����
A. ��ȥMgCl2������Һ�е�Fe3+�����Ƚ��裬����MgCO3�����ˣ�������������
B. �Ʊ�Fe(OH)3���壺��FeCl3��Һ�еμ�����NaOH��Һ
C. ����Fe2(SO4)3��Һ���Ƿ���FeSO4���μ��������Ը��������Һ������
D. ʹʢ������������þ�������Թ��еij����ܽ⣺����һ�������Ȼ����Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾϸ����ijЩ�л����Ԫ����ɺ��ܹ�ϵ������A��B����Ԫ�أ������������ӣ�ͼ��X��Y��Z��P�ֱ�Ϊ�����������ӵĻ�����λ����ش��������⣺

(1)ͼ��X��________��I��С����������Ҫ��ָ______________��
(2)ͼ��Z��________��ʹ�ü��̡�������(����)���ȾҺȾɫ����ʹ�����________ɫ��
(3)ͼ��P�ĽṹͨʽΪ________��д����P�γɢ��Ľṹ���______________��
(4)��͢����߶��ж����ԣ����߶����ԵĹ�ϵ��ǰ��________���ߡ�
(5)����ϸ���Ļ������У����������������Լ��ٵ���Ҫ��________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com