
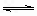 H2��������I2���������ﵽƽ��ʱ������˵����ȷ����
H2��������I2���������ﵽƽ��ʱ������˵����ȷ����  ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 9N2��g��+6CO2��g��+6H2O��g����
9N2��g��+6CO2��g��+6H2O��g���� 9N2��g��+12CO2��g��+12H2O��g����
9N2��g��+12CO2��g��+12H2O��g����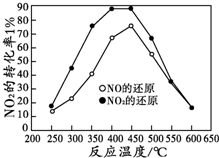
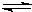 O2+Hb?CO K=220
O2+Hb?CO K=220�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ݻ���ͬ�������ܱ�������A��B�����¶�Ϊ423K����ͬʱ��A��B�м���amol��bmol��HI���壬��a>b������Ӧ��2HI������H2��������I2���������ﵽƽ��ʱ������˵����ȷ���� ( )
A���ӷ�Ӧ��ʼ���ﵽƽ�⣬����ʱ��t��A��>t��B����
B��ƽ��ʱI2��Ũ��c��I2��A��c��I2��B
C��ƽ��ʱI2�����ڻ�������е��������A�����еĴ���B����
D��HI�ķֽ��� ����A��������B��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣�(1)��ͬ�¶�ʱ����������ʼ�����ͬ�������зֱ����2molSO2��1molO2�����������ֺ�ѹ�����������ֺ��ݣ��ﵽƽ��ʱ��(����ڡ�С�ڡ������ڡ�)
��Ӧ����v��_____ v����SO2��ת���ʦ���_____����������ʱ��t��____t����
![]() (2)���ݻ���ͬ�������ܱ�����A��B��,�����¶�Ϊ423K����ͬʱ�ֱ���A��B�м���amol��2amol��HI����,����Ӧ:2HI(g) H2(g)��I2��g�����ﵽƽ��ʱ��(����ڡ�С�ڡ������ڡ�)
(2)���ݻ���ͬ�������ܱ�����A��B��,�����¶�Ϊ423K����ͬʱ�ֱ���A��B�м���amol��2amol��HI����,����Ӧ:2HI(g) H2(g)��I2��g�����ﵽƽ��ʱ��(����ڡ�С�ڡ������ڡ�)
I2��Ũ��c(I2)A____c(I2)B,,I2�����ڻ�������е��������A����_ _B����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ�Ž�һ�и߶���ѧ�ڵ�һ���¿�����ѧ�� ���ͣ������
��10�֣�(1)��ͬ�¶�ʱ����������ʼ�����ͬ�������зֱ����2molSO2��1molO2�����������ֺ�ѹ�����������ֺ��ݣ��ﵽƽ��ʱ��(����ڡ�С�ڡ������ڡ�)
��Ӧ����v��_____ v����SO2��ת���ʦ���_____����������ʱ��t��____t���� (2)���ݻ���ͬ�������ܱ�����A��B��,�����¶�Ϊ423K����ͬʱ�ֱ���A��B�м���amol��2amol��HI����,����Ӧ:2HI(g) H2(g)��I2��g�����ﵽƽ��ʱ��(����ڡ�С�ڡ������ڡ�)
(2)���ݻ���ͬ�������ܱ�����A��B��,�����¶�Ϊ423K����ͬʱ�ֱ���A��B�м���amol��2amol��HI����,����Ӧ:2HI(g) H2(g)��I2��g�����ﵽƽ��ʱ��(����ڡ�С�ڡ������ڡ�)
I2��Ũ��c(I2)A____c(I2)B, ,I2�����ڻ�������е��������A����_ _B����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���㽭ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
���ݻ���ͬ�������ܱ�������A��B�����¶�Ϊ423K����ͬʱ��A��B�м���amol��bmol��HI���壬��a>b������Ӧ��2HI������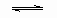 H2��������I2���������ﵽƽ��ʱ������˵����ȷ���� ( )
H2��������I2���������ﵽƽ��ʱ������˵����ȷ���� ( )
A���ӷ�Ӧ��ʼ���ﵽƽ�⣬����ʱ��t��A��>t��B�� ��
B��ƽ��ʱI2��Ũ��c��I2��A��c��I2��B
C��ƽ��ʱI2�����ڻ�������е��������A�����еĴ���B����
D��HI�ķֽ��� ����A��������B��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com