
���f�������һ��������ʹ��ҩ����Ĺ�ҵ�ϳ�·�����£�

 ��ش��������⣺
��ش��������⣺
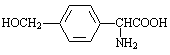
��A���ڱ�¶�ڿ����л���ʣ���ԭ���� ��
����A��B�ķ�Ӧͨ���ڵ��½��С��¶�����ʱ��������ȡ������������ࡣ���ж�����ȡ�����У���������ɵ��� ��������ĸ��
a��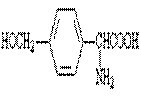 ����b��
����b��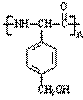 ����c��
����c��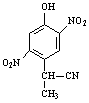 ����d��
����d��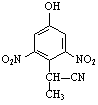
����E������ͬ���칹���У���������̼ԭ�ӵķ����� ��������ĸ��
a��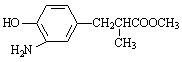 b��
b��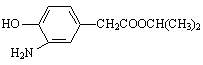
c��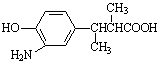 d��
d��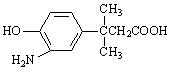
��F�Ľṹ��ʽ ��
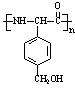
��D��ͬ���칹��H��һ�֦�-�����ᣬH�ɱ�����KMnO4��Һ�����ɶԱ������ᣬ��H�Ľṹ��ʽ�� ���߾���L��Hͨ���ļ����Ӷ��ɣ�L�Ľṹ��ʽ�� ��
���𰸡�
�ŷ�������ױ������е�O2���� ��a ��ac
��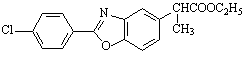
��
 ��
�� 
�����������ݺϳ�·��ͼ��E��F��G��ת����ϵ��E��G�Ľṹ�������Եó�F��GΪ������ˮ�ⷴӦ�����F�Ľṹ��ʽΪ 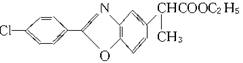
��ת����ϵ�ж�B��CΪ���ԭ������NO2����NH2����C��DΪ�����ữ���γɡ�COOH������CN����COOH����D��EΪ�������γɡ�COOC2H5������C��D�ֱ�Ϊ��
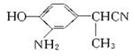
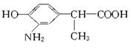
��C�� ��D��
��1��A�к��з��ǻ����ױ������е�O2������
��2���¶�����ʱ��������ȡ������������࣬��OH��Ӱ������Ҫ�ģ�ȡ��λ�����ڡ���λȡ��Ϊ���������п������ɵ���a��
��3������̼ԭ����ֱ��������4��ȡ����������ͬ��Ϊa��c����E������ͬ���칹���к�������̼ԭ�ӡ�
��5��H��D��ͬ���칹������һ�֦�-�����ᣬH�ɱ�����KMnO4��Һ�����ɶԱ������ᣬ˵��H���ڱ�����λ��������ȡ���������뱽��������̼ԭ������Hԭ�ӣ���H�Ľṹ��ʽΪ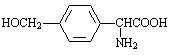 ��
��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪Co2O3��������Һ���ױ���ԭ��Co2+��Co2O3��Cl2��FeCl3��I2�����������μ��������з�Ӧ��ˮ��Һ����������������
A.3Cl2 + 6FeI2 == 2FeCl3 + 4FeI3
B.Cl2 + FeI2 == FeCl2 + I2
C.Co2O3 + 6HCl == 2CoCl2+ Cl2��+ 3H2O
D.2Fe3+ + 2I�� == 2Fe2+ + I2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ȱ������Ʊ�������֬����Ҫԭ�ϣ���ҵ���в�ͬ�ĺϳ�·�ߣ����������е�����(��Щ��Ӧδע������)��

���������գ�
(1)д����Ӧ���ͣ���Ӧ��___________________����Ӧ��__________________��
(2)д���ṹ��ʽ��X_______________________��Y_____ ______________��
______________��
(3)д����Ӧ�ڵĻ�ѧ����ʽ��___________________________��
(4)�뻷���ȱ��黥Ϊͬ���칹�壬�����ڴ��������(����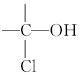 ��
�� �ṹ)��________________________�֡�
�ṹ)��________________________�֡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijЩ�߷��Ӵ����������л��ϳɡ�������һ�ָ߷��Ӵ����������ϳ�·�ߵ�һ���֣���͢����Ǣ��ĵ��壻��Ӧ����һ�������½��У��������͢��к�N�ӻ������������ڱ�������

�ش��������⣺
��1��д���ɻ������ϳɻ������ķ�Ӧ����ʽ____________________����Ҫ�� �����Ӧ��������
�����Ӧ��������
��2�����й��ڻ������͢��˵���У���ȷ����____________________������ĸ����
A.���������Է���������Ӧ
B.�������������Ʒ�Ӧ����������
C.���������Է���ˮ�ⷴӦ
D.�������������ʹ������Ȼ�̼��Һ��ɫ
E.�����������ϩ�������
��3�����������_______________������ĸ������
A.�� B.���� C.ϩ�� D.�� E.��
��4��д��2�ֿɼ�����͢��Ļ�ѧ�Լ�_____________��
��5���������ϳ�·���У���������͢��ڴ�������������������Ӧ���ɢ���ˮ��д����Ӧ����ʽ________________________����Ҫ������Ӧ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��A��B��C��D��E��F�� G��H��Ϊ�л������
G��H��Ϊ�л������
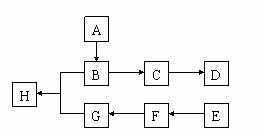
�ش��������⣺
��1���л�������A����Է�������С��60��A�ܷ����۾���Ӧ��1 mol A�ڴ�������������3 mol H2��Ӧ����B����A�Ľṹ��ʽ�� ����A����B�ķ�Ӧ������ ��
��2��B��Ũ�����м��ȿ�����C��C�ڴ��������¿ɾۺ����ɸ߷��ӻ�����D����C����D�Ļ�ѧ����ʽ�� ��
��3���ٷ��㻯����E�ķ���ʽ��C8H8Cl2��E�ı����ϵ�һ��ȡ����ֻ��һ�֣���E�����п��ܵĽṹ��ʽ��
��E��NaOH��Һ�п�ת��ΪF��F�ø������������Һ��������G��C8H6O4����
1 mol G��������NaHCO3��Һ��Ӧ�ɷų�44.8 L CO2����״�������ɴ�ȷ��E�Ľṹ��ʽ�� ��
��4��G��������B��Ũ������¼��ȷ�Ӧ������H������G��B����H�Ļ�ѧ����ʽ�� ���÷�Ӧ�ķ�Ӧ������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������һ��ֻ��C��H��O�Ļ�����A��A���������ϣ�����Է�������Ϊ88��������C��H��Oԭ �Ӹ�����Ϊ2��4��1��
�Ӹ�����Ϊ2��4��1��
��1��A�ķ���ʽΪ ��
��2��д����A����ʽ��ͬ���������Ľṹ��ʽ��
��
��֪����ROH+HBr�������ᣩ RBr+H2O
RBr+H2O


A�к���̼��˫������A��صķ�Ӧ���£�
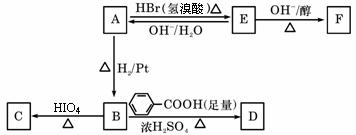
��3��д��A��E��E��F�ķ�Ӧ ���ͣ�A��E ��E��F ��
���ͣ�A��E ��E��F ��
��4��д��A��C��F�Ľṹ��ʽ��A ��C ��F ��
��5��д��B��D��Ӧ�Ļ�ѧ����ʽ��
��6���ڿ����г�ʱ��������ͣ�A��ת��Ϊ��Է�������Ϊ86�Ļ�����G��G��һ�� ����ֻ��һ�֣�д��G�Ľṹ��ʽ�� ��A��G�ķ�Ӧ����Ϊ ��
����ֻ��һ�֣�д��G�Ľṹ��ʽ�� ��A��G�ķ�Ӧ����Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪�� ��ע��R��R��Ϊ������AΪ�л��ϳ��м��壬��һ�������·�����ȥ��Ӧ�����ܵõ����ֻ�Ϊͬ���칹��IJ�����е�һ��B��������ȡ�ϳ���֬��Ⱦ�ϵȶ��ֻ�����Ʒ��A�ܷ�������ͼ��ʾ�ı仯��
��ע��R��R��Ϊ������AΪ�л��ϳ��м��壬��һ�������·�����ȥ��Ӧ�����ܵõ����ֻ�Ϊͬ���칹��IJ�����е�һ��B��������ȡ�ϳ���֬��Ⱦ�ϵȶ��ֻ�����Ʒ��A�ܷ�������ͼ��ʾ�ı仯��
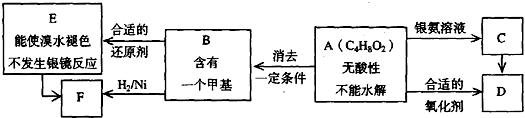
�Իش�
(1)д��������������A��ͬ���칹��ṹ��ʽ(����дһ��)��
a.��������______________________
b.�ܷ���ˮ�ⷴӦ______________________
(2)A�����еĹ�������___________��D�Ľṹ��ʽ��_____________��
(3)C��D�ķ�Ӧ������___________��E��F�ķ�Ӧ������___________
a.������Ӧ b.��ԭ��Ӧ c.�ӳɷ�Ӧ d.ȡ����Ӧ
(4)д����ѧ����ʽ��A��B________________________________��
(5)д��E���ɸ߾���Ļ�ѧ����ʽ��___________________________________��
(6)C��ͬ���칹��C1��C����ͬ�����ţ�������C1��ȥ������ˮ�γɺ�����Ԫ����C2��д��C2�Ľṹ��ʽ��___________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����AlCl3�� ��KClO��HClO2��NaClO3��Cl2O7��һ���������У�������Ӧ����������ǣ� ����
A��HCl B��Cl2 C��Ca(ClO)2 D��Cl2O3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵������ȷ�ǣ�NA��ʾ�����ӵ������� �� ��
A��4��ʱ��5��4mLˮ��������ˮ��������0��3NA
B���ڳ��³�ѹ�£�11��2L����������ԭ����ΪNA
C����״���£�22��4L����������������Ϊ2 2��4NA
D��2L 1mol��L-1K2SO4��Һ�����ӵ�����Ϊ3 NA
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com