
 �ǻ���ϩ�Ľṹ��ʽ���ɽ�һ����дΪ
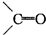
�ǻ���ϩ�Ľṹ��ʽ���ɽ�һ����дΪ ����ϩ���Ļ�ѧ���ʸ�ϩ�����ƣ� ���л��������е�ϩ�����Ը�������O3����Ӧ������п�۴�����ˮ�⼴��ԭ�е�ϩ�����ѣ����Ѵ����˵�̼ԭ�Ӹ����1����ԭ�Ӷ�����ȩ����-CHO����ͪ����
����ϩ���Ļ�ѧ���ʸ�ϩ�����ƣ� ���л��������е�ϩ�����Ը�������O3����Ӧ������п�۴�����ˮ�⼴��ԭ�е�ϩ�����ѣ����Ѵ����˵�̼ԭ�Ӹ����1����ԭ�Ӷ�����ȩ����-CHO����ͪ���� ������������Ӧ����һ�𣬳�Ϊ��ϩ���ij����ֽ⡱�����磺
������������Ӧ����һ�𣬳�Ϊ��ϩ���ij����ֽ⡱�����磺



 �ṹ�������������ֽ����л������к����ʻ���
�ṹ�������������ֽ����л������к����ʻ��� ��b mol����a��b�Ĵ�����ϵ�ǣ�
��b mol����a��b�Ĵ�����ϵ�ǣ� �����Ҵ�Ϊ�л�ԭ�ϣ��ϳɼ�����������ĸ�����Ӧ����ʽ
�����Ҵ�Ϊ�л�ԭ�ϣ��ϳɼ�����������ĸ�����Ӧ����ʽ ��
�� ��
�� ��
��
 ��
�� ��
�� ��
��
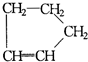
 ��A���������ò���B��B�Ļ�ѧʽΪC10H20���������ݱ�����B�����ں�����Ԫ̼������д��A��B�Ľṹ��ʽA
��A���������ò���B��B�Ļ�ѧʽΪC10H20���������ݱ�����B�����ں�����Ԫ̼������д��A��B�Ľṹ��ʽA



 �����Ʋ��[A]�ĽṹʽΪ
�����Ʋ��[A]�ĽṹʽΪ 

 �����ݷ���ʽ��֪�������ʵ���֮��Ϊ2��1��
�����ݷ���ʽ��֪�������ʵ���֮��Ϊ2��1�� �ṹ�������л���Ϊ��״��ϩ����ϩ�����ݴ˽����Ϣ���
�ṹ�������л���Ϊ��״��ϩ����ϩ�����ݴ˽����Ϣ��� ������ȥ��Ӧ�������Ļ���ϩ������ϩ�������O3����Ӧ������п�۴�����ˮ������OHCCH2CH2CH2CH2CHO��������������ͭ��Ӧ����HOOCCH2CH2CH2CH2COOH��HOOCCH2CH2CH2CH2COOH���Ҵ���1��2��Ӧ���ɼ������������
������ȥ��Ӧ�������Ļ���ϩ������ϩ�������O3����Ӧ������п�۴�����ˮ������OHCCH2CH2CH2CH2CHO��������������ͭ��Ӧ����HOOCCH2CH2CH2CH2COOH��HOOCCH2CH2CH2CH2COOH���Ҵ���1��2��Ӧ���ɼ������������ ��
��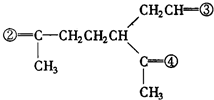 �����������ں͢��Ƚӳɻ����ٺ͢��ٽ�Ϊ�����������γ���Բ�����������⣬�ۢܽӳɻ������ں͢۽ӳɻ����ٺ͢��ٽ�Ϊ�����������γ���Բ�����������⣬�ݴ˽��
�����������ں͢��Ƚӳɻ����ٺ͢��ٽ�Ϊ�����������γ���Բ�����������⣬�ۢܽӳɻ������ں͢۽ӳɻ����ٺ͢��ٽ�Ϊ�����������γ���Բ�����������⣬�ݴ˽�� ���֪�������д�����Ԫ����
���֪�������д�����Ԫ����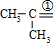 ��
��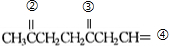 ���ڢ������γ���Ԫ�����٢������γɲ������ݴ���д��
���ڢ������γ���Ԫ�����٢������γɲ������ݴ���д��| O3 |
| Zn/H2O |
| O3 |
| Zn/H2O |
| O3 |
| Zn/H2O |
| m |
| M |
| O3 |
| Zn/H2O |
| O3 |
| Zn/H2O |
 ���ɷ���ʽ��֪HCHO��
���ɷ���ʽ��֪HCHO�� �������ʵ���֮��Ϊ2��1��
�������ʵ���֮��Ϊ2��1�� ��2��1��
��2��1�� �ṹ������Ϊ��״��ϩ�������Ӻ���2��C=C˫�����ɷ�Ӧ��Ϣ��֪��1mol������������4mol
�ṹ������Ϊ��״��ϩ�������Ӻ���2��C=C˫�����ɷ�Ӧ��Ϣ��֪��1mol������������4mol ����b=4a����Ϊ��ϩ���������к���1��C=C˫������1mol������������2mol
����b=4a����Ϊ��ϩ���������к���1��C=C˫������1mol������������2mol ����b=2a���ʴ�Ϊ��b=4a��b=2a��
����b=2a���ʴ�Ϊ��b=4a��b=2a�� ������ȥ��Ӧ�������Ļ���ϩ������ϩ�������O3����Ӧ������п�۴�����ˮ������OHCCH2CH2CH2CH2CHO��������������ͭ��Ӧ����HOOCCH2CH2CH2CH2COOH��HOOCCH2CH2CH2CH2COOH���Ҵ���1��2��Ӧ���ɼ�������������ϳɼ�����������ĸ�����Ӧ����ʽΪ��
������ȥ��Ӧ�������Ļ���ϩ������ϩ�������O3����Ӧ������п�۴�����ˮ������OHCCH2CH2CH2CH2CHO��������������ͭ��Ӧ����HOOCCH2CH2CH2CH2COOH��HOOCCH2CH2CH2CH2COOH���Ҵ���1��2��Ӧ���ɼ�������������ϳɼ�����������ĸ�����Ӧ����ʽΪ�� ��
�� ��
�� ��
�� ��
�� ��
�� ��
�� ��
�� ��
�� ��
�� �����������ں͢��Ƚӳɻ����ٺ͢��ٽ�Ϊ�����������γ���Բ�����������⣬�ۢܽӳɻ������ں͢۽ӳɻ����ٺ͢��ٽ�Ϊ�����������γ���Բ�����������⣬��AΪ
�����������ں͢��Ƚӳɻ����ٺ͢��ٽ�Ϊ�����������γ���Բ�����������⣬�ۢܽӳɻ������ں͢۽ӳɻ����ٺ͢��ٽ�Ϊ�����������γ���Բ�����������⣬��AΪ ��BΪ
��BΪ ��
�� ��
�� ��
�� ���֪�������д�����Ԫ����
���֪�������д�����Ԫ����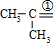 ��
��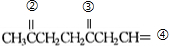 ���ڢ������γ���Ԫ�����٢������γɲ�������AΪ
���ڢ������γ���Ԫ�����٢������γɲ�������AΪ ��
�� ��
��| O3 |
| Zn/H2O |
| O3 |
| Zn/H2O |
| O3 |
| Zn/H2O |
| 8.7g |
| 58g/mol |
| O3 |
| Zn/H2O |


 ���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д�
���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д� ���Ͱ�ͨ������ϵ�д�
���Ͱ�ͨ������ϵ�д� �ٷ�ѧ����ҵ��������ϵ�д�
�ٷ�ѧ����ҵ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�043
�ɱ���������������Ϣ�������ѧ֪ʶ���ش��������⣺
����֪�л������е�ϩ���ɷ��������ֽⷴӦ�����磺
![]()
��![]() �������з���õ����ɰش��������ʽΪC10H12O3�����Ȳ�����ˮ��Ҳ������NaHCO3��Һ����ͼ��ʾΪ�ɰش��IJ������ʡ�
�������з���õ����ɰش��������ʽΪC10H12O3�����Ȳ�����ˮ��Ҳ������NaHCO3��Һ����ͼ��ʾΪ�ɰش��IJ������ʡ�
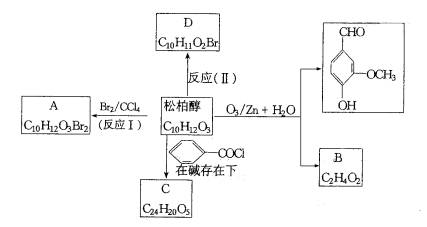
�Իش𣺣�1��д��������Ľṹ��ʽ���ɰش�����������������B����������������C����������������D����������������
��2��д����Ӧ���ͣ���Ӧ����������������������Ӧ��������������������
��3��д����Ӧ�������Ļ�ѧ����ʽ���л���Ҫд�ṹ��ʽ����������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
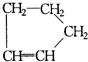

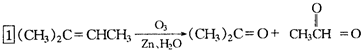
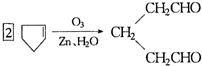
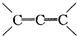

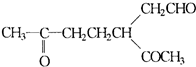
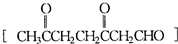
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009���㽭ʡ����������һ�и�����ѧģ���Ծ��������棩 ���ͣ������
 �ǻ���ϩ�Ľṹ��ʽ���ɽ�һ����дΪ
�ǻ���ϩ�Ľṹ��ʽ���ɽ�һ����дΪ ����ϩ���Ļ�ѧ���ʸ�ϩ�����ƣ� ���л��������е�ϩ�����Ը�������O3����Ӧ������п�۴�����ˮ�⼴��ԭ�е�ϩ�����ѣ����Ѵ����˵�̼ԭ�Ӹ����1����ԭ�Ӷ�����ȩ����-CHO����ͪ����
����ϩ���Ļ�ѧ���ʸ�ϩ�����ƣ� ���л��������е�ϩ�����Ը�������O3����Ӧ������п�۴�����ˮ�⼴��ԭ�е�ϩ�����ѣ����Ѵ����˵�̼ԭ�Ӹ����1����ԭ�Ӷ�����ȩ����-CHO����ͪ���� ������������Ӧ����һ�𣬳�Ϊ��ϩ���ij����ֽ⡱�����磺
������������Ӧ����һ�𣬳�Ϊ��ϩ���ij����ֽ⡱�����磺

 �ṹ�������������ֽ����л������к����ʻ���
�ṹ�������������ֽ����л������к����ʻ��� ��b mol����a��b�Ĵ�����ϵ�ǣ� �� ��
��b mol����a��b�Ĵ�����ϵ�ǣ� �� �� �����Ҵ�Ϊ�л�ԭ�ϣ��ϳɼ�����������ĸ�����Ӧ����ʽ ��
�����Ҵ�Ϊ�л�ԭ�ϣ��ϳɼ�����������ĸ�����Ӧ����ʽ �� ��A���������ò���B��B�Ļ�ѧʽΪC10H20���������ݱ�����B�����ں�����Ԫ̼������д��A��B�Ľṹ��ʽA ��B ��
��A���������ò���B��B�Ļ�ѧʽΪC10H20���������ݱ�����B�����ں�����Ԫ̼������д��A��B�Ľṹ��ʽA ��B �� �����Ʋ��[A]�ĽṹʽΪ
�����Ʋ��[A]�ĽṹʽΪ �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
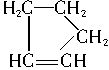 �ǻ���ϩ�Ľṹ��ʽ���ɽ�һ����дΪ
�ǻ���ϩ�Ľṹ��ʽ���ɽ�һ����дΪ ������ϩ�Ļ�ѧ���ʸ�ϩ�������ơ�
������ϩ�Ļ�ѧ���ʸ�ϩ�������ơ�
 �������ֽ����ø��ֲ���Ľṹ��ʽ�������ʵ���֮�ȡ�
�������ֽ����ø��ֲ���Ľṹ��ʽ�������ʵ���֮�ȡ� ���ϳɼ���ȩ��
���ϳɼ���ȩ�� ���ĸ���ת����ϵ��
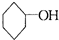
���ĸ���ת����ϵ�� ��A��������ò���B��B�ķ���ʽΪC10H20���������ݱ���������B�ں���һ����Ԫ̼������д��A��B�Ľṹ��ʽ������ע�����ƣ���AΪ____��BΪ_______��
��A��������ò���B��B�ķ���ʽΪC10H20���������ݱ���������B�ں���һ����Ԫ̼������д��A��B�Ľṹ��ʽ������ע�����ƣ���AΪ____��BΪ_______���鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com