

���� װ�â����Ʊ��õ�ClO2�����Ԣ��з�ӦΪNaClO3��Na2SO3��ŨH2SO4���������� ClO2��Na2SO4��װ�â�Ϊ��ȫƿ��װ�âܷ�Ӧ�����Һ���NaClO2���壬װ�â�������NaClO2��ClԪ�صĻ��ϼ۽��ͣ�˫��ˮӦ���ֻ�ԭ�ԣ����������ɣ�װ�â�Ϊ���ն�������壬��ֹ��Ⱦ������װ�â������բ���ʣ�����壬
��1����װ�������������װ�������ԵIJ����ǣ����ȹرշ�Һ©����������K1��K2��Ȼ����١����м�ˮû��������¶ˣ��þƾ�����������ƿ�����١��ݴ������ݣ�ֹͣ���ȣ�һ��ʱ��١��ݸ�������γ�һ���ȶ���ˮ����˵������װ�����������ã�
��2������������;ѡȡ������
��3��װ��C�������ǰ�ȫƿ���з��������ã�
��4��װ�â���ClO2���������ơ�˫��ˮ����NaClO2��ClԪ�صĻ��ϼ۽��ͣ�˫��ˮӦ���ֻ�ԭ�ԣ����������ɣ��ݴ���д��
��5������Ŀ��Ϣ��֪��Ӧ�����¶�38�桫60�棬����60��ʱNaClO2�ֽ��NaClO3��NaCl��
��6����װ��D����Һ���NaClO2���壬��Ҫ�����ᾧ�����ȹ��ˡ�ϴ�ӡ����
��7�����п��ܷ���Na2SO3+H2SO4��Ũ��=Na2SO4+SO2��+H2O��������SO2 �������װ���У�SO2��H2O2 ��Ӧ���������ƣ�
��8���ٵ������۱���ɫ����Ӧ����ʱ���ⷴӦ��ȫ���μ����һ��Na2S2O3��Һʱ��Һ��ɫǡ����ȥ�Ұ�����ڲ���ԭ��˵������ζ��յ㣻
�ڸ��ݻ�ѧ��Ӧ�ɵù�ϵʽ��NaClO2��2I2��4S2O32-������Ʒ��NaClO2�����ʵ���x�����ݹ�ϵʽ���㣮
��� �⣺װ�â����Ʊ��õ�ClO2�����Ԣ��з�ӦΪNaClO3��Na2SO3��ŨH2SO4���������� ClO2��Na2SO4��װ�â�Ϊ��ȫƿ��װ�âܷ�Ӧ�����Һ���NaClO2���壬װ�â�������NaClO2��ClԪ�صĻ��ϼ۽��ͣ�˫��ˮӦ���ֻ�ԭ�ԣ����������ɣ�װ�â�Ϊ���ն�������壬��ֹ��Ⱦ������װ�â������բ���ʣ�����壬
��1����װ�������������װ�������ԵIJ����ǣ����ȹرշ�Һ©����������K1��K2��Ȼ����١����м�ˮû��������¶ˣ��þƾ�����������ƿ�����١��ݴ������ݣ�ֹͣ���ȣ�һ��ʱ��١��ݸ�������γ�һ���ȶ���ˮ����˵������װ�����������ã�
�ʴ�Ϊ���رշ�Һ©��������
��2����50%˫��ˮ����30%��H2O2��Һ����Ҫ����������Ͳ���ձ���������������ιܣ����Ի���Ҫ��Ͳ��
�ʴ�Ϊ����Ͳ��
��3��װ��C�������ǰ�ȫƿ����ֹDƿ��Һ������Bƿ�У�
�ʴ�Ϊ����ȫƿ����������
��4��װ�â���ClO2���������ơ�˫��ˮ����NaClO2��ClԪ�صĻ��ϼ۽��ͣ�˫��ˮӦ���ֻ�ԭ�ԣ����������ɣ���ҺΪ������Һ�����������ӷ���ʽΪ��2ClO2+2OH-+H2O2=2ClO2-+O2+2H2O��
�ʴ�Ϊ��2ClO2+2OH-+H2O2=2ClO2-+O2+2H2O��
��5������Ŀ��Ϣ��֪��Ӧ�����¶�38�桫60�棬����60��ʱNaClO2�ֽ��NaClO3��NaCl�����������ȥD�е���ˮԡ�����ܵ��²�Ʒ�л��е�������NaClO3��NaCl��
�ʴ�Ϊ��NaClO3��NaCl��
��6����װ��D����Һ���NaClO2���壬��Ҫ�����ᾧ�����ȹ��ˡ�ϴ�ӡ�������������Ե�iii��������38��-60����ˮ�ز�����ע��©��û����������ˮ��Ȼ�������ٴμ�ˮ���ظ�2-3�Σ�
�ʴ�Ϊ����38��-60����ˮ�ز�����ע��©��û����������ˮ��Ȼ�������ٴμ�ˮ���ظ�2-3�Σ�
��7�����п��ܷ���Na2SO3+H2SO4��Ũ��=Na2SO4+SO2��+H2O��������SO2 �������װ���У�SO2��H2O2 ��Ӧ���������ƣ�Ũ�����ѻӷ������������ѻӷ����Σ���������װ�ã���a��ȷ��b��c����
�ʴ�Ϊ��a��
��8���ٵ������۱���ɫ����Ӧ����ʱ���ⷴӦ��ȫ���μ����һ��Na2S2O3��Һʱ��Һ��ɫǡ����ȥ�Ұ�����ڲ���ԭ��
�ʴ�Ϊ���μ����һ��Na2S2O3��Һʱ����Һ��ɫǡ����ȥ�Ұ�����ڲ���ԭ��
������Ʒ��NaClO2�����ʵ���x����
NaClO2��2I2��4S2O32-
1mol 4mol
0.25x c mol•L-1��V��10-3L
��x=c•V•10-3mol��
�ʴ�Ϊ��c•V•10-3mol��
���� ���⿼�����������Ʊ�ʵ��Ļ����������������Ƶ����ʼ��к͵ζ���֪ʶ������ԭ���ǽ���Ĺؼ���ͬʱ����ѧ���������⡢���������������ѵ�������ͼ�ķ�������Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ij��Һ�м�����������Һʱ�����ɰ�ɫ������˵����Һ����Cl- | |
| B�� | ij��Һ�м���ϡ������Һʱ��������ɫ���壬˵����Һ����CO32- | |
| C�� | ij��Һ�м����Ȼ�����Һʱ�����ɰ�ɫ������˵����Һ����SO42- | |
| D�� | ij��Һ�м���������Һ�������������ټ����Ȼ�����Һʱ�����ɰ�ɫ������˵����Һ����SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
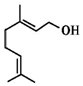
| A�� | ����ʹ������Ȼ�̼��Һ��ɫ | B�� | ��Ҷ���ķ���ʽΪC10H20O | ||
| C�� | ����ʹ���Ը��������Һ��ɫ | D�� | �ܷ���ȡ����Ӧ�ͼӳɷ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.1mol NaHCO3�������NA��CO32- | |
| B�� | ��״���£�11.2L��18O2��������������Ϊ8NA | |
| C�� | 1mol Fe��������ϡHNO3��Ӧ��ת�Ƶ�����ĿΪ3NA | |
| D�� | ��״���£�22.4L���к���̼�����ĿΪ6NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 �ס��ҡ�������������ת����ϵ��ͼ��ʾ����Ӧ������ȥ����ͷ��ʾһ��ת���������и��������У�������ͼʾת����ϵ���ǣ�������
�ס��ҡ�������������ת����ϵ��ͼ��ʾ����Ӧ������ȥ����ͷ��ʾһ��ת���������и��������У�������ͼʾת����ϵ���ǣ�������| �� | �� | �� | �� | |
| A | Cu | FeCl3��Һ | ��CuCl2��Һ | Fe |
| B | H2O | Fe | H2 | O2 |
| C | CH3CH2OH | O2 | CH3CHO | H2 |
| D | SiO2 | Na2CO3 | Si | O2 |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʯ�͵��ѽ� | B�� | ú�ĸ��� | C�� | ʯ�͵��ѻ� | D�� | ʯ�͵ķ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������Ӽ������������Ӽ������ۼ������������������������������Ӽ����ۼ��ǻ�ѧ�� | |
| B�� | ʯīϩ��һ�ִ�ʯī�������á�˺�ѡ�����������ĵ���̼ԭ������ϣ������ַ�����C60�����ʯ���л�á�ֻ��һ��̼ԭ�Ӻ��̼��Ƭ��Ҳ�ؽ���Ϊ�о����� | |
| C�� | �������ʵ��۷е��ɴ�С���У�����裾̼���裾���ʯ | |
| D�� | ��ɫ����̼�������۵�2200�棬����̬�����磬��ԭ�Ӿ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
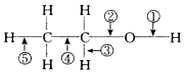
| A�� | ��Ũ�����ϼ�����170�棬�Ϣڢ� | B�� | ����±�ᷴӦ�Ϣ� | ||
| C�� | ���Ӽ���ˮ�Ϣٻ�� | D�� | ����ᷴӦ�Ϣ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڱ�״���£�22.4L ˮ����������ĿΪ NA | |
| B�� | 1mol•L-1K2SO4��Һ���� K+��ĿΪ 2NA | |
| C�� | 1 mol ����������Ӧ���� Na2O �� Na2O2ʱ��ʧ������Ŀ��Ϊ NA | |
| D�� | O2 ��Ħ�����ԼΪ 22.4 L•mol-1 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com