
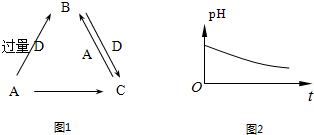
 ����ӦA+D����������C�Ƕ�����̼������������Һ��Ӧ����Ӧ�����ӷ���ʽΪCO2+2OH-=CO32-+H2O��
����ӦA+D����������C�Ƕ�����̼������������Һ��Ӧ����Ӧ�����ӷ���ʽΪCO2+2OH-=CO32-+H2O��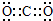 ��CO2+2OH-=CO32-+H2O��
��CO2+2OH-=CO32-+H2O��

 ��һ����ͬ���ɽ�����ϵ�д�
��һ����ͬ���ɽ�����ϵ�д� ������Ӧ���ϵ�д�
������Ӧ���ϵ�д� ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 A��B��C��D��Ϊ�������ʣ��֮��Ĺ�ϵ��ͼ��ʾ����-����ʾ�������ʼ��ܷ�����Ӧ����������ʾ���ʼ��ת����ϵ�����ַ�Ӧ����������Լ���Ӧ��������ȥ����
A��B��C��D��Ϊ�������ʣ��֮��Ĺ�ϵ��ͼ��ʾ����-����ʾ�������ʼ��ܷ�����Ӧ����������ʾ���ʼ��ת����ϵ�����ַ�Ӧ����������Լ���Ӧ��������ȥ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
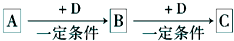 A��B��C��D��Ϊ��ѧ��ѧ�г��������ʣ�����֮���ת����ϵ����ͼ�����ֲ�������ȥ�����Իش�
A��B��C��D��Ϊ��ѧ��ѧ�г��������ʣ�����֮���ת����ϵ����ͼ�����ֲ�������ȥ�����Իش�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 A��B��C��D��Ϊ������Ԫ�أ�A��B��ͬ�������ڵ�����Ԫ�أ�A��C��ͬ�������ڵ�����Ԫ�أ�A��B��C����Ԫ�ص�ԭ������֮��Ϊ31��DԪ����A��B��C����Ԫ�ؼȲ���ͬ���ڣ�Ҳ��ͬ���壮��ش�
A��B��C��D��Ϊ������Ԫ�أ�A��B��ͬ�������ڵ�����Ԫ�أ�A��C��ͬ�������ڵ�����Ԫ�أ�A��B��C����Ԫ�ص�ԭ������֮��Ϊ31��DԪ����A��B��C����Ԫ�ؼȲ���ͬ���ڣ�Ҳ��ͬ���壮��ش�

 NH3?H2O+H+
NH3?H2O+H+ NH3?H2O+H+
NH3?H2O+H+ 2NH3
2NH3 2NH3
2NH3

| 4(m-n) |
| 5 |
| 4(m-n) |
| 5 |
| 4n+m |
| 5 |
| 4n+m |
| 5 |
| 4m+3n |
| 5 |
| 4m+3n |
| 5 |
| m-3n |
| 5 |
| m-3n |
| 5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
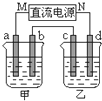 ��ͼ��ʾ��װ���У��ס������ձ��зֱ�ʢ����������CuSO4��Һ��100g 10.00%��K2SO4��Һ��a��b��c��d��Ϊʯī�缫����ͨ��Դһ��ʱ��������K2SO4��ҺŨ��Ϊ10.47%������a�缫���������ӣ�����˵����ȷ���ǣ�������
��ͼ��ʾ��װ���У��ס������ձ��зֱ�ʢ����������CuSO4��Һ��100g 10.00%��K2SO4��Һ��a��b��c��d��Ϊʯī�缫����ͨ��Դһ��ʱ��������K2SO4��ҺŨ��Ϊ10.47%������a�缫���������ӣ�����˵����ȷ���ǣ�������| A���ס�����Һ��pH����С | B���缫b��������������ԼΪ2.8L����״���£� | C���缫d�Ϸ����ķ�ӦΪ��2H2O+2e-?H2��+2OH- | D����ʹ���е���Һ�ָ���ԭ����Ũ�ȣ��ɼ���24.5g��Cu��OH��2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
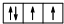 ���ͱ����γ����ֻ�����X��Y��X��ˮ��Ӧ������һ�־��л�ԭ�ԵĶ�Ԫ��M��1mol�����������ɻ�������Z�����õ�Z����ˮ��Ӧ�IJ���W����12mol KOH������ȫ�кͣ�������������ȼ�����ɻ�����N��N��ˮ��Ӧ����W��DԪ�ص���̬�⻯��Իش��������⣺
���ͱ����γ����ֻ�����X��Y��X��ˮ��Ӧ������һ�־��л�ԭ�ԵĶ�Ԫ��M��1mol�����������ɻ�������Z�����õ�Z����ˮ��Ӧ�IJ���W����12mol KOH������ȫ�кͣ�������������ȼ�����ɻ�����N��N��ˮ��Ӧ����W��DԪ�ص���̬�⻯��Իش��������⣺�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com