
�����������ε�ϡ��Һ���ֱ���a mol��L��1 NaX��Һ��b mol��L��1 NaY��Һ������˵������ȷ����
A����a��b��pH(NaX)��pH(NaY)������ͬŨ��ʱ������HX��HY
B����a��b�������c(X��)��c(Y��)��c(HY) ������ͬŨ��ʱ������HX��HY
C����a��b�����c(X��)��c(Y��)������Ƴ���Һ��c(HX)��c(HY)������ͬŨ��ʱ������HX��HY
D��������Һ�������ϣ����c(X��)��c(Y��)��c(HX)��c(HY)��0.1 mol��L��1 ������Ƴ�a+b��0.2 mol��L��1 ����������Һ���ʱ��Һ����ĸı䣩
��������
���������Aѡ����������ҺŨ����ͬ��PH��NaX��> PH��NaY��,NaX�ļ��Ը�ǿ����Ӧ��X-��ˮ��̶ȸ�ǿ���������HX<HY��Bѡ����a��b����c(X��)��c(Y��)��c(HY)����˿��Կ���X-û��ˮ�⣬���Ϊǿ�ᣬ������HX��HY��Cѡ����a��b����c(X��)��c(Y��)�����Ե�֪X-ˮ��IJ��ִ���Y��ˮ��IJ��֣�����c(HX)��c(HY)������ͬŨ��ʱ������HX��HY��Dѡ�������������ϣ����Ϻ������ǻ��ǰ������������c(X��)��c(Y��)��c(HX)��c(HY)=��a+b��/2,����Ϊc(X��)��c(Y��)��c(HX)��c(HY)��0.1mol/L�����Եõ�a+b��0.2mol/L��
���㣺����ˮ��
���������⽫����ˮ��Ļ���֪ʶ������ۺϿ��죬������Ҫ��Ƚϸߣ�����һ���Ѷ���Ŀ��


 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A.��a=b,pH��NaX����pH(NaY)������ͬŨ��ʱ������HX��HY
B.��a=b�������c(X-)=c(Y-)+c(HY)����HX��ǿ�ᣬHY������
C.��a��b�����c(X-)=c(Y-)������Ƴ���Һ��c(HX)��c(HY)������ͬŨ��ʱ������HX��HY
D.��������Һ�������ϣ����c(X-)+c(Y-)+c(HX)+c(HY)=0.1 mol��L-1������Ƴ�a=b=0.1 mol��L-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A.��a=b��pH(NaX)��pH(NaY)������ͬŨ��ʱ������HX��HY
B.��a=b�������c(X-)=c(Y-)+c(HY)������ͬŨ��ʱ������HX��HY
C.��a��b�����c(X-)=c(Y-)������Ƴ���Һ��c(HX)��c(HY)������ͬŨ��ʱ������HX��HY
D.������Һ�������ϣ����c(X-)+c(Y-)+c(HX)+c(HY)=0.1 mol��L-1������Ƴ�a=b=0.1 mol��L-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������������һ�и߶���ѧ�����п������ƻ�ѧ�Ծ����������� ���ͣ���ѡ��
�����������ε�ϡ��Һ���ֱ���a mol��L��1 NaX��Һ��b mol��L��1 NaY��Һ������˵������ȷ����
| A����a��b��pH(NaX)��pH(NaY)������ͬŨ��ʱ������HX��HY |
| B����a��b�������c(X��)��c(Y��)��c(HY) ������ͬŨ��ʱ������HX��HY |
| C����a��b�����c(X��)��c(Y��)������Ƴ���Һ��c(HX)��c(HY)������ͬŨ��ʱ������HX��HY |
| D��������Һ�������ϣ����c(X��)��c(Y��)��c(HX)��c(HY)��0.1 mol��L��1������Ƴ�a+b��0.2 mol��L��1 ����������Һ���ʱ��Һ����ĸı䣩 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ��ӱ�ʡ������ѧ�ڵ��Ĵ��¿���ѧ�Ծ� ���ͣ�ѡ����
�����������ε�ϡ��Һ���ֱ���a mol��LNaX��Һ��b mol��LNaY��Һ������˵������ȷ���� �� ��
A����a=b����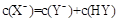 ������ͬ����ʱ������
������ͬ����ʱ������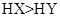
B����a=b����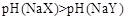 ������ͬ����ʱ������
������ͬ����ʱ������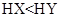
C����a>b����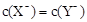 ������ͬ����ʱ������HX<HY
������ͬ����ʱ������HX<HY
D�����������ϣ���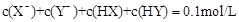 ����һ����
����һ����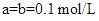
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com