
�� ���� ���� | IA | | 0 | |||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | | | | �� | �� | �� | | |
| 3 | �� | | �� | �� | | | �� | |
 ��3���ڡ��ۡ��ߵ���ۺ������������ǿ������˳����_________________�����ѧʽ��
��3���ڡ��ۡ��ߵ���ۺ������������ǿ������˳����_________________�����ѧʽ�� ��4���١��ܡ��ݡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д������һ�ֻ�����Ļ�ѧʽ��____________________��
��4���١��ܡ��ݡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д������һ�ֻ�����Ļ�ѧʽ��____________________�� ��7���ɱ�������Ԫ�ص�ԭ�Ӱ�1:1��ɵij���Һ̬�������ϡҺ�ױ�MnO2���ֽ⣬д����Ӧ�Ļ�ѧ����ʽ_________________��
��7���ɱ�������Ԫ�ص�ԭ�Ӱ�1:1��ɵij���Һ̬�������ϡҺ�ױ�MnO2���ֽ⣬д����Ӧ�Ļ�ѧ����ʽ_________________�� ��У���˿��ֿ���ϵ�д�
��У���˿��ֿ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������







�� ���� ���� | IA | | 0 | |||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | | | | �� | �� | �� | | |
| 3 | �� | | �� | �� | | | �� | |
 ��2���ڡ��ۡ��ߵ���ۺ������������ǿ������˳���� ��
��2���ڡ��ۡ��ߵ���ۺ������������ǿ������˳���� �� ��3���١��ܡ��ݡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д������һ�ֻ�����ĵ���ʽ�� ��
��3���١��ܡ��ݡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д������һ�ֻ�����ĵ���ʽ�� �� ��4���ɱ�������Ԫ�ص�ԭ�Ӱ�1�U1��ɵij���Һ̬�������ϡҺ�ױ����ֽ⣬��ʹ�õĴ���Ϊ������ţ� _��
��4���ɱ�������Ԫ�ص�ԭ�Ӱ�1�U1��ɵij���Һ̬�������ϡҺ�ױ����ֽ⣬��ʹ�õĴ���Ϊ������ţ� _�� a��MnO2 b��FeCl3 c��Na2SO3 d��KMnO4
a��MnO2 b��FeCl3 c��Na2SO3 d��KMnO4 ��5���ɱ���Ԫ���γɵij�������X��Y��Z��M��N�ɷ������·�Ӧ��
��5���ɱ���Ԫ���γɵij�������X��Y��Z��M��N�ɷ������·�Ӧ��
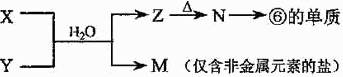
 X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪ ��
X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪ �� N���ĵ��ʵĻ�ѧ����ʽΪ ��
N���ĵ��ʵĻ�ѧ����ʽΪ �� �����£�Ϊʹ0.1 mol/L M ��Һ����M���������������Ũ����ȣ�Ӧ����Һ�м���һ������Y��Һ�� ��
�����£�Ϊʹ0.1 mol/L M ��Һ����M���������������Ũ����ȣ�Ӧ����Һ�м���һ������Y��Һ�� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
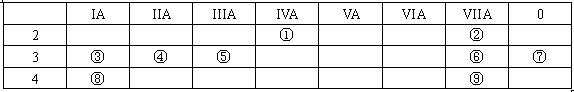 ��1�����Ԫ�ط�����__________
��1�����Ԫ�ط�����__________�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ����������� ���������� ������������� ������
���е��������� ���������� ������������� ������ �γɵĻ�����ĵ���ʽ ���˻��������� ������ӻ�������ۻ��������
�γɵĻ�����ĵ���ʽ ���˻��������� ������ӻ�������ۻ���������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢� | B���ڢ� | C���٢� | D���ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
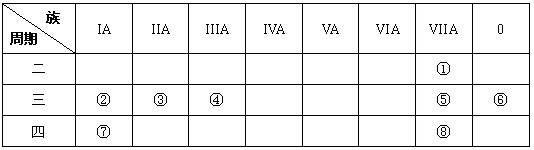
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��O2��O3 |
B��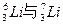 |
| C��C2H6��C2H4 |
D��CH3��CH2��CH2��CH3�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com