��1����ط�Ӧͨ���Ƿ��ȷ�Ӧ�����з�Ӧ�������Ͽ���Ƴ�ԭ��صĻ�ѧ��Ӧ��
������ţ������෴Ӧ�߱��������Ǣ�
��Ӧ����
��Ӧ��
A��C��s��+H
2O��g���TCO��g��+H
2��g����H��0
B��Ba��OH��
2?8H
2O��s��+2NH
4Cl��s���TBaCl
2��aq��+2NH
3?H
2O�� 1��+8H
2O�� 1��
��H��0
C��CaC
2��s��+2H
2O�� 1���TCa��OH��
2��s��+C
2H
2��g����H��0
D��CH
4��g��+2O
2��g���TCO
2��g��+2H
2O�� 1����H��0
��2����KOH��ҺΪ�������Һ�����ݣ�I����ѡ��Ӧ���һ����أ��为����ӦΪ��
��
��3�����ԭ���ڻ�ѧ��ҵ���й㷺��Ӧ�ã��ֽ�����Ƶ�ԭ���ͨ��������ͼ�е�������������aΪ���Һ��X��Y�������缫��
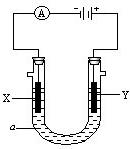
����X��Y��Ϊ���Ե缫��aΪ����ʳ��ˮ������ʱ����Y�缫��Ӧ����ķ�����
��
����X��Y�ֱ�Ϊʯī������a��Ϊ���͵�NaCl��Һ��������������ɵİ�ɫ����¶���ڿ����У��ɹ۲쵽��������
��
����X��Y��Ϊ���Ե缫��aΪһ��Ũ�ȵ�����ͭ��Һ��ͨ��������ܷ�Ӧ��ѧ����ʽΪ
��ͨ��һ��ʱ�����������Һ�м���0.05mol Cu��OH��
2��ǡ�ûָ����ǰ��Ũ�Ⱥ�pH����������е���ת�Ƶ����ʵ���Ϊ
mol��
��4�����ù�ҵ�����ӽ���Ĥ�����ռ��ԭ��������ͼ��ʾװ�õ��K
2SO
4��Һ��
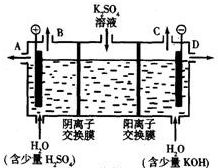
�ٸõ��۵�������ӦʽΪ
��ͨ�������ӽ���Ĥ��������
�����������������=����ͨ�������ӽ���Ĥ����������
��ͼ��a��b��c��d�ֱ��ʾ�й���Һ��pH����a��b��c��d��С�����˳��Ϊ
��
�۵��һ��ʱ���B����C�ڲ��������������Ϊ
��

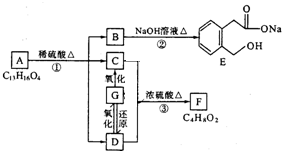 ͼ��A��B��C��D��E��F��G��Ϊ�л������B������ֻ��һ����״�ṹ������ͼʾ�ش����⣺
ͼ��A��B��C��D��E��F��G��Ϊ�л������B������ֻ��һ����״�ṹ������ͼʾ�ش����⣺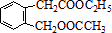
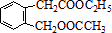

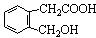 �����F�ķ���ʽC4H8O2��C��Dת��ΪF�ķ�Ӧ����������֪CΪ���ᣬDΪ�Ҵ���GΪ��ȩ��FΪ������������BCD�Ľṹ�����A�ķ���ʽ��Aת��ΪBCD�ķ�Ӧ���������Ƴ�AΪ
�����F�ķ���ʽC4H8O2��C��Dת��ΪF�ķ�Ӧ����������֪CΪ���ᣬDΪ�Ҵ���GΪ��ȩ��FΪ������������BCD�Ľṹ�����A�ķ���ʽ��Aת��ΪBCD�ķ�Ӧ���������Ƴ�AΪ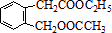 ����ϣ��л���Ľṹ�������Լ���ĿҪ��ͽ�����
����ϣ��л���Ľṹ�������Լ���ĿҪ��ͽ�����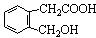 �����F�ķ���ʽC4H8O2��C��Dת��ΪF�ķ�Ӧ����������֪CΪ���ᣬDΪ�Ҵ���GΪ��ȩ��FΪ������������BCD�Ľṹ�����A�ķ���ʽ��Aת��ΪBCD�ķ�Ӧ���������Ƴ�AΪ
�����F�ķ���ʽC4H8O2��C��Dת��ΪF�ķ�Ӧ����������֪CΪ���ᣬDΪ�Ҵ���GΪ��ȩ��FΪ������������BCD�Ľṹ�����A�ķ���ʽ��Aת��ΪBCD�ķ�Ӧ���������Ƴ�AΪ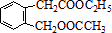 ��
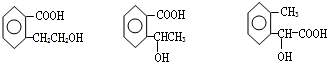
��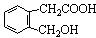 ������ʽΪC9H10O3��A�Ľṹ��ʽ��
������ʽΪC9H10O3��A�Ľṹ��ʽ��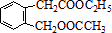 ����Ӧ��Ϊ����ˮ�ⷴӦ��
����Ӧ��Ϊ����ˮ�ⷴӦ��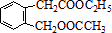 ��ˮ�⣻
��ˮ�⣻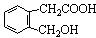 ����Ӧ��ͬ���칹���У��ٺ����ڶ�ȡ�������ṹ��
����Ӧ��ͬ���칹���У��ٺ����ڶ�ȡ�������ṹ��  ��������ͬ���칹�壬
��������ͬ���칹�壬


 Сѧ�̲�ȫ��ϵ�д�
Сѧ�̲�ȫ��ϵ�д� Сѧ��ѧ������ѿڶ���ϵ�д�
Сѧ��ѧ������ѿڶ���ϵ�д� ������Ӧ�������������ϵ�д�
������Ӧ�������������ϵ�д� �㽭֮�ǿ�ʱ�Ż���ҵϵ�д�
�㽭֮�ǿ�ʱ�Ż���ҵϵ�д�

 +2NaOH
+2NaOH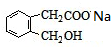 +CH3COONa+CH3CH2OH
+CH3COONa+CH3CH2OH +2NaOH
+2NaOH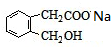 +CH3COONa+CH3CH2OH
+CH3COONa+CH3CH2OH

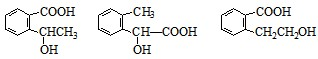 ������һ��
������һ��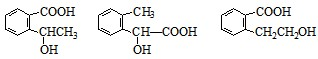 ������һ��
������һ�� ����CH2=CH2+H2O
����CH2=CH2+H2O ����CH2=CH2+H2O
����CH2=CH2+H2O ������ͼ�ش����⣺
������ͼ�ش����⣺

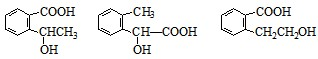 ������һ��
������һ��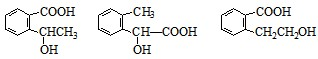 ������һ��
������һ��
