
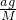 ��
��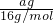 =4��11��
=4��11��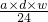 mol��
mol��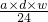 mol=
mol=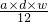 mol��
mol��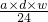 mol��
mol��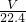 mol������Ϊ
mol������Ϊ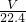 mol��17g/mol=
mol��17g/mol=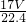 g��
g��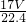 g+1000g�������V=
g+1000g�������V=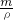 =
=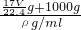 =
=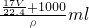 ����
����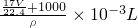 ��
�� =
=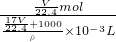 =
=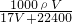 mol/L��
mol/L��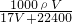 mol/L��
mol/L�� mol��
mol�� mol��
mol�� ������������ʵ�����������ͬ�¶Ⱥ�����£������ѹǿ֮�ȵ������ʵ���֮�ȼ���A��Ħ��������
������������ʵ�����������ͬ�¶Ⱥ�����£������ѹǿ֮�ȵ������ʵ���֮�ȼ���A��Ħ��������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
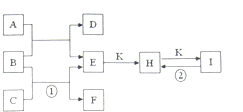 ��ͼ�dz�����һЩ���ʼ��仯����֮���ת����ϵͼ�����³�ѹ�£�D��F��K��Ϊ��ɫ�̼�����ζ�����壬CΪ���õĽ������ʣ�B���������ɫҺ�壬A���е���C��D��ȼ�����ɵĵ���ɫ���壬I�DZ��Ƹ�����õķ��ͷ۵���Ҫ�ɷ�֮һ������Ӧ�����ɵIJ�����������ȥ��
��ͼ�dz�����һЩ���ʼ��仯����֮���ת����ϵͼ�����³�ѹ�£�D��F��K��Ϊ��ɫ�̼�����ζ�����壬CΪ���õĽ������ʣ�B���������ɫҺ�壬A���е���C��D��ȼ�����ɵĵ���ɫ���壬I�DZ��Ƹ�����õķ��ͷ۵���Ҫ�ɷ�֮һ������Ӧ�����ɵIJ�����������ȥ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com