
��1����֪����![]()
![]()
![]()
��![]() ��
��![]() =__________________________��
=__________________________��
(2)��ҵ�ϳɵ��ķ�ӦΪ![]() ����һ���¶��£���һ������N
����һ���¶��£���һ������N![]() ��H
��H![]() ͨ�뵽���Ϊ1L���ܱ������дﵽƽ��ı�������������ʹƽ��������Ӧ�����ƶ���ƽ�ⳣ���������___________________��
ͨ�뵽���Ϊ1L���ܱ������дﵽƽ��ı�������������ʹƽ��������Ӧ�����ƶ���ƽ�ⳣ���������___________________��
������ѹǿ ������Ӧ���Ũ��
��ʹ�ô��� �ܽ����¶�
(3) ��ҵ�ϳɰ��ķ�ӦΪ![]() �������ݻ�Ϊ2.0L���ܱ������г���
�������ݻ�Ϊ2.0L���ܱ������г���![]() ��
��![]() ����Ӧ��һ�������´ﵽƽ��ʱ��NH
����Ӧ��һ�������´ﵽƽ��ʱ��NH![]() �����ʵ�������(NH
�����ʵ�������(NH![]() �����ʵ����뷴Ӧ��ϵ���ܵ����ʵ���֮��)Ϊ
�����ʵ����뷴Ӧ��ϵ���ܵ����ʵ���֮��)Ϊ![]() ������
������
�ٸ�������N![]() ��ƽ��ת����Ϊ______________��
��ƽ��ת����Ϊ______________��
�ڸ����·�Ӧ![]() ��ƽ�ⳣ��Ϊ_____________��
��ƽ�ⳣ��Ϊ_____________��
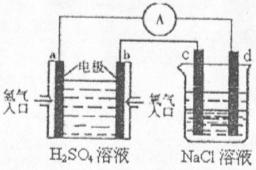
(4)�ϳɰ���ԭ��������һ�����͵���ɫ��Դ�����й����ķ�չǰ������������ȼ�ϵ�ؽ�����ͼ��ʾʵ�飺(����c��d��Ϊ̼����NaCI��Һ�����Ϊ500m1)
��a��Ϊ________�����缫��Ӧʽ__________________________��
c��Ϊ________�����缫��Ӧʽ__________________________
����ͼװ���У���b�������ĵ�O![]() �ڱ�״���µ����Ϊ280mlʱ����NaCl��Һ
�ڱ�״���µ����Ϊ280mlʱ����NaCl��Һ
��PHΪ_________ (���跴Ӧǰ����Һ������䣬��NaCl��Һ����)
 ���ĺ����Ͼ�������ϵ�д�
���ĺ����Ͼ�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2011?����ģ�⣩�£�N2H4���ֳ���������һ�ֿ�ȼ��Һ�壬��ȼ���Ƚϴ���ȼ�ղ���Ի�������Ⱦ���ʿ����������ȼ�ϣ�
��2011?����ģ�⣩�£�N2H4���ֳ���������һ�ֿ�ȼ��Һ�壬��ȼ���Ƚϴ���ȼ�ղ���Ի�������Ⱦ���ʿ����������ȼ�ϣ� N2H+5+OH-��N2H+5+H2O
N2H+5+OH-��N2H+5+H2O N2H+6+OH-
N2H+6+OH- N2H+5+OH-��N2H+5+H2O
N2H+5+OH-��N2H+5+H2O N2H+6+OH-
N2H+6+OH-�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2012?����һģ����Դ����������������ٵ��ش���⣬�ձ����������ĺ�й©�¹����������ǶԺ���Դ�Ŀֻţ����״���δ����Ҫ����ɫ��Դ֮һ��
��2012?����һģ����Դ����������������ٵ��ش���⣬�ձ����������ĺ�й©�¹����������ǶԺ���Դ�Ŀֻţ����״���δ����Ҫ����ɫ��Դ֮һ��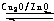 CH3OH��g�����״������ʵ����뷴Ӧ�¶ȵĹ�ϵ��ͼ��ʾ��
CH3OH��g�����״������ʵ����뷴Ӧ�¶ȵĹ�ϵ��ͼ��ʾ��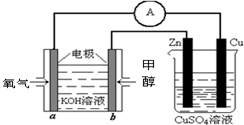
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� ����ʽ��HCl ��Է���������36.5 �ܶȣ�1.19g?cm-3HCl������������36.5%�ٸ�Ũ������HCl�����ʵ���Ũ��Ϊ 11.9 11.9 mol/L���ڱ�״���£�1.00Lˮ���ܶȣ�1.00g?cm-3������ 353 353 L��HCl���Ƶ�����Ũ���ᣮ
�鿴�𰸺ͽ���>> ͬ����ϰ��� ����ѧУ��ѡ - ��ϰ���б� - �����б� ����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר�� Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com��Ȩ��������վ�������£�ͼƬ��Դ�����磬����Ȩ����Ȩ��ԭ�������У�ת�������ַ���Ȩ��������Ȩ����������������֪�����ǽ����촦������ϵqq��3310059649�� ICP�������: ��ICP��07509807��-10 ����������42018502000812�� |