
����С������14�֣�ij�о���ѧϰС������֤���ȵ�̿��Ũ���ᷢ����Ӧ�����ɵĸ��ֲ��������������������������ʵ�����̣�
��1�����з�Ӧ�Ļ�ѧ����ʽΪ ���������������� ������ ��
��2���ڴ��������ǣ���������������������������������������������
��3��Ʒ����Һʹ�������Σ���һ��ʹ��ʱ��������������������������������
�ڶ���ʹ��ʱ��������________________ �������� _��
��4����������Ҳ��ʹ��ˮ��ɫ�������˶�������� �� �ԡ�������ԭ������Ư�ס���
��Ӧ�Ļ�ѧ����ʽΪ������������������������������������������
��5������������������������������������������֤��������һ����CO2����
��1��C+ 2H2SO4(Ũ) CO2��+ 2SO2��+ 2H2O
��2����ɫ�������ɫ
��3�������Ƿ���SO2���ɣ�������SO2�Ƿ����
��4����ԭ����SO2 + Br2 + 2H2O== H2SO4 + 2HBr
��5������Ʒ����Һ����ɫ�����г���ʯ��ˮ����ǡ�
����:��1��Ũ�������ǿ�����ԣ��ڼ���ʱ���Ժ�̼��Ӧ����CO2��SO2�����Լ�ˮ������
��2�����ɵ�ˮ������ɫ����ͭ�����ɵ�����������ɫ��
��3��SO2����Ư���ԣ���ʹƷ����Һ��ɫ�����Կ���Ʒ��������SO2�Ĵ��ڡ���ΪSO2Ҳ��ʹ�����ʯ��ˮ����ǣ������ڼ���CO2֮ǰ��Ҫ���ȳ�ȥSO2��Ϊ�˼���SO2�Ƿ���ȫ�����գ���Ҫ��һ��ͨ��Ʒ����Һ��
��4����ΪSO2���л�ԭ�ԣ���ˮ���������ԣ����Կ�����ˮ������SO2��
��5��ֻ�Т��е�Ʒ����Һ������ɫ������֤��SO2�Ѿ���ȫ�����գ������ʱ�����ʯ��ˮ����ǣ������˵��һ����CO2���ɡ�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�긣��ʡ�ϰ�һ�и�һ��ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
����С������14�֣�a��b��c��d��e���ֶ�����Ԫ�ص�ԭ������������aΪ�ǽ���Ԫ�أ���a��eͬ���壬c��dΪͬ���ڵ�����Ԫ�ء�eԭ�ӵ�����������c��dԭ������������֮�͡�bԭ���������������ڲ��������2����c���⻯���������3�����ۼ������ƶϣ�
��1��д��bԪ�������ڱ��е�λ�� ��
��2����a��c��d���γɵ����ӻ������� ����e������������Ӧˮ�������Һ����ʱ��Ӧ�����ӷ���ʽ�� ��
��3��c�ĵ��ʵĵ���ʽΪ ��
��4��b��d��ȣ��ǽ����Խ�ǿ���� ����Ԫ�ط��ű�ʾ����������ʵ��֤����һ���۵��� ��ѡ����ĸ��ţ���
| A�������£���ĵ��ʳʹ�̬����ĵ��ʳ���̬ |
| B������⻯����ȶ���ǿ�ڣ���⻯�� |
| C��������γɵĻ������У�������� |
| D������⻯��ķе���ڣ���⻯�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�긣��ʡ�ϰ�һ�и�һ��ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ�ʵ����
����С������14�֣�ij�о���ѧϰС������֤���ȵ�̿��Ũ���ᷢ����Ӧ�����ɵĸ��ֲ��������������������������ʵ�����̣�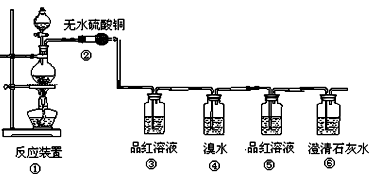
��1�����з�Ӧ�Ļ�ѧ����ʽΪ ���������������� ������ ��
��2���ڴ��������ǣ���������������������������������������������
��3��Ʒ����Һʹ�������Σ���һ��ʹ��ʱ��������������������������������
�ڶ���ʹ��ʱ��������________________ �������� _��
��4����������Ҳ��ʹ��ˮ��ɫ�������˶�������� �� �ԡ�������ԭ������Ư�ס���
��Ӧ�Ļ�ѧ����ʽΪ������������������������������������������
��5������������������������������������������֤��������һ����CO2����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�츣��ʡ��һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
����С������14�֣�a��b��c��d��e���ֶ�����Ԫ�ص�ԭ������������aΪ�ǽ���Ԫ�أ���a��eͬ���壬c��dΪͬ���ڵ�����Ԫ�ء�eԭ�ӵ�����������c��dԭ������������֮�͡�bԭ���������������ڲ��������2����c���⻯���������3�����ۼ������ƶϣ�
��1��д��bԪ�������ڱ��е�λ�� ��
��2����a��c��d���γɵ����ӻ������� ����e������������Ӧˮ�������Һ����ʱ��Ӧ�����ӷ���ʽ�� ��
��3��c�ĵ��ʵĵ���ʽΪ ��
��4��b��d��ȣ��ǽ����Խ�ǿ���� ����Ԫ�ط��ű�ʾ����������ʵ��֤����һ���۵��� ��ѡ����ĸ��ţ���
���������£���ĵ��ʳʹ�̬����ĵ��ʳ���̬
�£�����⻯����ȶ���ǿ�ڣ���⻯��
�ã�������γɵĻ������У��������
�ģ�����⻯��ķе���ڣ���⻯��
��5��Ԫ��b�γɵ�һ�ֵ��ʾ���Ӳ�ȴ��۵�ߵ��������ʣ��ݴ��жϸõ��ʵľ�������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�콭��ʡ��һ��һѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
����С������14�֣�ij�о���ѧϰС������֤���ȵ�̿��Ũ���ᷢ����Ӧ�����ɵĸ��ֲ��������������������������ʵ�����̣�
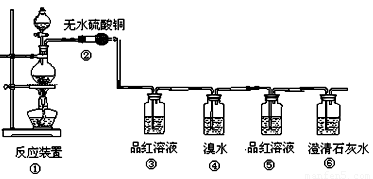
��1�����з�Ӧ�Ļ�ѧ����ʽΪ ���������������� ������ ��
��2���ڴ��������ǣ���������������������������������������������
��3��Ʒ����Һʹ�������Σ���һ��ʹ��ʱ��������������������������������
�ڶ���ʹ��ʱ��������________________ �������� _��
��4����������Ҳ��ʹ��ˮ��ɫ�������˶�������� �� �ԡ�������ԭ������Ư�ס���
��Ӧ�Ļ�ѧ����ʽΪ������������������������������������������
��5������������������������������������������֤��������һ����CO2����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com