
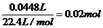 ��ת�Ƶ��ӵ����ʵ�����0.04mol����������M��+1�ۣ����Ե�ת��0.04mol����ʱ����0.04mol�������ʣ�M=
��ת�Ƶ��ӵ����ʵ�����0.04mol����������M��+1�ۣ����Ե�ת��0.04mol����ʱ����0.04mol�������ʣ�M=


 �����ҵ��ٿ���������������ϵ�д�
�����ҵ��ٿ���������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
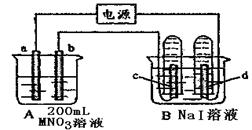
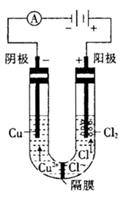
| A��b�缫�Ϸ���������Ӧ |
| B����װ���ܽ���ѧ��ת��ɵ��� |
| C���������Һ��Cu2+��b�缫��a�缫Ǩ�� |
| D����aΪͭ����a�ĵ缫��ӦʽΪ��Cu-2e-=Cu2+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

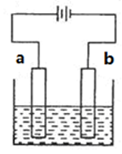
 ������ȫ������pH��3.2ʱ
������ȫ������pH��3.2ʱ
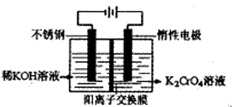
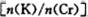 Ϊd�����ʱ�ĸ���ص�ת����Ϊ ��
Ϊd�����ʱ�ĸ���ص�ת����Ϊ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
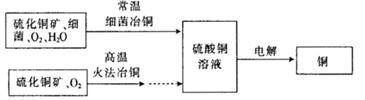
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
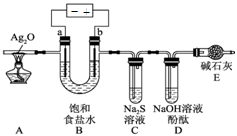
 6Cu+SO2������Ӧ���������� ��
6Cu+SO2������Ӧ���������� ��
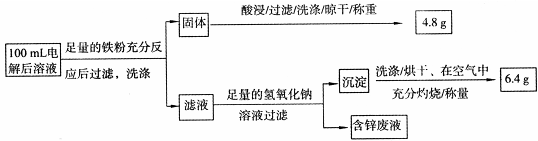
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
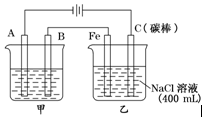
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��2��7 mol�� L-1 | B��3 mol�� L-1 | C��4 mol�� L-1 | D��1 mol�� L |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com