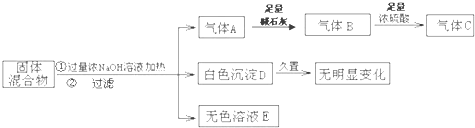
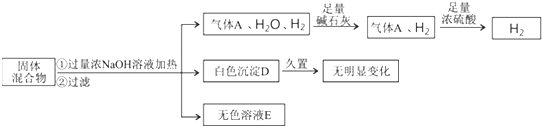
����⣺������Ũ�������Ʒ�Ӧ����������A��ͨ����ʯ�Ҹ�����پ���Ũ���ᣬŨ������������˵������NH
3����һ������NH
4Cl������Ũ�����ʣ������C����˵������H
2��һ������Al���õ���ɫ����D���Ҿ�������ɫ�仯��˵������MgCl
2��������FeCl
2�����ɳ���ΪMg��0H��
2��ʵ�鲻��ȷ���Ƿ���Al
2��SO
4��
3��
��1����ʯ�Ҿ�����ˮ�ԣ����������������е�ˮ����������Ũ�����ʣ������C����˵������H
2��һ������Al����Ӧ�����ӷ���ʽΪ2Al+2OH
-+2H
2O=2AlO
2-+3H
2����
�ʴ�Ϊ����������A���������е�ˮ������2Al+2OH
-+2H
2O=2AlO
2-+3H
2����
��2��ͨ����ʯ�Ҹ�����پ���Ũ���ᣬŨ������������˵������NH
3����һ������NH
4Cl��
�ʴ�Ϊ������B����Ũ�����Ũ������������˵��NH
3 �����գ�������к���NH
4Cl��
��3����ɫ����D��������ɫ�仯��˵������MgCl
2��������FeCl
2����������������������Ѹ�ٱ�ɻ���ɫ�����ձ�ɺ��ɫ��
�ʴ�Ϊ����ɫ����D������ɫ�����Ա仯��˵��������FeCl
2��
��4���õ���ɫ����D���Ҿ�������ɫ�仯��˵������MgCl
2��������FeCl
2�����ɳ���ΪMg��0H��
2������MgCl
2�����ɳ���ΪMg��0H��
2��������þԪ���غ�õ���������ɫ����D��Mg��OH��
2����������Ϊ5.80g�����ʵ���=
=0.1mol�����Ȼ�þ������=0.1mol��95g/mol=9.5g��
�ʴ�Ϊ��MgCl
2��9.5��
��5��ʵ�����û���漰Al
2��SO
4��
3�����ʼ���Ӧ������ȷ���Ƿ���Al
2��SO
4��
3�����鷽��Ϊȡ������ɫ��ҺE���Թ��У��ȵμ�ϡ��������Һ�����ԣ��ٵμ�BaCl
2��Һ���۲��Ƿ��а�ɫ�������ɣ����а�ɫ������֤������Al
2��SO
4��
3��
�ʴ�Ϊ��Al
2��SO
4��
3��ȡ������ɫ��ҺE���Թ��У��ȵμ�ϡ��������Һ�����ԣ��ٵμ�BaCl
2��Һ���۲��Ƿ��а�ɫ�������ɣ����а�ɫ������֤������Al
2��SO
4��
3��