
��10�֣�ijУ��ѧ�о���ѧϰС����������˽�������ݣ��Ҷ���(HOOC-COOH���ɼ�дΪH2C2O4)�׳Ʋ��ᣬ������ˮ�����ڶ�Ԫ��ǿ��(Ϊ�������)��������ǿ��̼�ᣬ���۵�Ϊ101.5�棬��157��������Ϊ̽������IJ��ֻ�ѧ���ʣ�����������ʵ�飺
(1)��ʢ��1 mL����NaHCO3��Һ���Թ��м��������Ҷ�����Һ���۲쵽����ɫ���ݲ������÷�Ӧ�����ӷ���ʽΪ___________________________________��
(2)��ʢ���Ҷ��ᱥ����Һ���Թ��е��뼸�������ữ��KMnO4��Һ������������Һ���Ϻ�ɫ��ȥ��˵���Ҷ������_____________(������ԡ�������ԭ�ԡ������ԡ�)������ƽ�÷�Ӧ�����ӷ���ʽ��
____ MnO4�C + ____ H2C2O4 + ____ H+ =" ____" Mn2+ + ____ CO2�� + ____ H2O
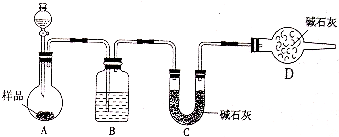
(3)��һ�������Ҷ�������Թ��У�����ͼ��ʾװ�ý���ʵ��(ע�����Բ�����������Ҽг�װ��δ���)��
ʵ�鷢�֣�װ��C��G�г���ʯ��ˮ����ǣ�B��CuSO4��ĩ������F��CuO��ĩ�к�ɫ��Ϊ��ɫ���ݴ˻ش�
����װ���У�D��������__________________.
�Ҷ���ֽ�Ļ�ѧ����ʽΪ______________________________________.
(4)��С��ͬѧ��2.52 g���ᾧ��(H2C2O4��2H2O)���뵽100 mL 0.2 mol/L��NaOH��Һ�г�ַ�Ӧ����÷�Ӧ����Һ�����ԣ�������Һ����仯���������й�ϵ��������
A��c(Na+)+c(H+)=c(HC2O4��)+c(OH��)+c(C2O42-)
B��c(HC2O4-)+c(C2O42-)="0.2" mol��L-1
C�� c(HC2O4-)>c(C2O42��)>c(H2C2O4)
D��c(Na+)=c(H2C2O4)+c(HC2O4-)+c(C2O42��)
(1)HCO3�C + H2C2O4 = HC2O4�C+ CO2��+ H2O(2��)
(2) ��ԭ��(1��) 2 5 6 2 10 8��2��)
(3) ��ȥ��������е�CO2 (1��)�� H2C2O4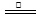 H2O+CO��+CO2�� (2��)
H2O+CO��+CO2�� (2��)
(4) C D(2��)
�������������(1)��ɫ����Ϊ������̼��HCO3�C + H2C2O4 = HC2O4�C+ CO2��+ H2O
��2������KMnO4Ϊǿ����������ɫ˵���Ҷ�����л�ԭ�ԡ��ɵ�ʧ�����غ��Ԫ���غ���ƽ��
��3���������Ƶ�����Ϊ��ȥ��������е�CO2��װ��C��G�г���ʯ��ˮ����ǣ�˵���ж�����̼��B��CuSO4��ĩ������˵����ˮ������F��CuO��ĩ�к�ɫ��Ϊ��ɫ��˵����һ����̼���ֽⷽ��ʽ��CO2H2C2O4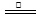 H2O+CO��+CO2�� ��4����Ӧ������ԣ��ɵ� c(HC2O4-)>c(C2O42��)>c(H2C2O4)�������������ӵ���غ�;c(Na+)=c(H2C2O4)+c(HC2O4-)+c(C2O42��).
H2O+CO��+CO2�� ��4����Ӧ������ԣ��ɵ� c(HC2O4-)>c(C2O42��)>c(H2C2O4)�������������ӵ���غ�;c(Na+)=c(H2C2O4)+c(HC2O4-)+c(C2O42��).
���㣺̽����ѧ��Ӧ����������ʵ�鷽�������
������������һ��ʵ��̽���⣬����ѧ�������ͽ�������������ע��ƽʱֻ�ǵĻ����ǽ���Ĺؼ����ۺ��Խ�ǿ���Ѷȴ�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д� Ŀ�����ϵ�д�
Ŀ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ijУ��ѧ�о���ѧϰС���������ʵ�鷽�����ⶨ���ü��õ�С�մ���Ʒ�д��������������
ijУ��ѧ�о���ѧϰС���������ʵ�鷽�����ⶨ���ü��õ�С�մ���Ʒ�д���������������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��������������е�����ѧ������ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��14�֣�ijУ��ѧ�о���ѧϰС���������ʵ�鷽�����ⶨ�����Ѿõ�С�մ���Ʒ�д��������������
��1������һ����ȡһ����������Ʒ�����������м��������غ���ȴ������ʣ��������������㡣ʵ���м��������ص�Ŀ���� ��
��2������������ȡһ������Ʒ������С�ձ��У�������ˮ�ܽ⣬��С�ձ��м��������Ȼ�����Һ������ϴ�ӣ���������������������������㣺
�ٹ��˲����У������ձ���©����õ��IJ���������______________________��
���������жϳ����Ƿ���ȫ�ķ�����_______________________________________
���������Լ���Ϊ������������֪�Ƶ���Ʒ9.5g������ij�������Ϊ19.7g������Ʒ��̼���Ƶ���������Ϊ_________________������һλС������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��������������и�����ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��14�֣�ijУ��ѧ�о���ѧϰС���������ʵ�鷽�����ⶨ�����Ѿõ�С�մ���Ʒ�д��������������
��1������һ����ȡһ����������Ʒ�����������м��������غ���ȴ������ʣ��������������㡣ʵ���м��������ص�Ŀ���� ��
��2������������ȡһ������Ʒ������С�ձ��У�������ˮ�ܽ⣬��С�ձ��м��������Ȼ�����Һ������ϴ�ӣ���������������������������㣺
�ٹ��˲����У������ձ���©����õ��IJ���������______________________��
���������жϳ����Ƿ���ȫ�ķ�����_______________________________________
���������Լ���Ϊ������������֪�Ƶ���Ʒ9.5g������ij�������Ϊ19.7g������Ʒ��̼���Ƶ���������Ϊ_________________������һλС������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com