
��8�֣���Ȼ������Ҫ�ɷּ���ȼ�����ɶ�����̼��Һ̬ˮ���Ȼ�ѧ����ʽ�ǣ�
CH4(g) + 2O2(g) == CO2(g) + 2H2O��l�� ��H== ��889.6kJ/mol
��ش��������⣺
��1����Ӧ�������ܺ�________������ڡ�����С�ڡ����ڡ��������������ܺ͡�
��2����1 mol������ȫȼ�����ɶ�����̼��ˮ��������ų������� ���������������������889.6kJ��
��3����֪����ȼ������Һ̬ˮ���Ȼ�ѧ����ʽ�ǣ�2H2(g)+O2(g) ===2H2O��l�� ��H =��572kJ/mol ������ͬ�����ļ������������ȫȼ������Һ̬ˮ�����Ƚ϶����________��
��4����ͼ��ʾ��װ������CH4 ��O2��KOH��Һ��ɵ�����ȼ�ϵ�أ����ø�װ�ÿ��Խ� ��ת��Ϊ �ܡ�
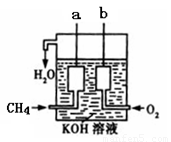
(8��)��1�����ڣ�2�֣� ��2��<��2�֣�
��3��������2�֣� ��4����ѧ�ܣ����ܣ�ÿ�ո�1�֣�
����������1������ȼ���Ƿ��ȷ�Ӧ�����Է�Ӧ�������ܺʹ��������������ܺ͡�
��2��������̬ˮ����������Һ̬ˮ����������˼���ȼ������Һ̬ˮ�ų��������ࡣ������1 mol������ȫȼ�����ɶ�����̼��ˮ��������ų���������889.6kJ��
��3�������Ȼ�ѧ����ʽ��֪����λ�����ļ����������ȫȼ�շų��������ֱ���889.6kJ��16��55.6kJ��572kJ��4��143kJ����������ȼ�շų��������ࡣ
��4��ԭ����ǰѻ�ѧ��ת��Ϊ���ܵ�װ�á�


 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��Ȼ������Ҫ�ɷּ���ȼ�����ɶ�����̼��Һ̬ˮ���Ȼ�ѧ����ʽ���£���ش��������⣺CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-889.6kJ/mol��
��Ȼ������Ҫ�ɷּ���ȼ�����ɶ�����̼��Һ̬ˮ���Ȼ�ѧ����ʽ���£���ش��������⣺CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-889.6kJ/mol���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�긣��ʡ��ɽ�صڶ���ѧ�߶���һ���¿���ѧ�Ծ����������� ���ͣ������
��8�֣���Ȼ������Ҫ�ɷּ���ȼ�����ɶ�����̼��Һ̬ˮ���Ȼ�ѧ����ʽ�ǣ�
CH4(g) + 2O2(g) == CO2(g) + 2H2O��l�� ��H="=" ��889.6kJ/mol
��ش��������⣺
��1����Ӧ�������ܺ�________������ڡ�����С�ڡ����ڡ��������������ܺ͡�
��2����1 mol������ȫȼ�����ɶ�����̼��ˮ��������ų������� ���������������������889.6kJ��
��3����֪����ȼ������Һ̬ˮ���Ȼ�ѧ����ʽ�ǣ�2H2(g)+O2(g) ===2H2O��l�� ��H =��572kJ/mol ������ͬ�����ļ������������ȫȼ������Һ̬ˮ�����Ƚ϶����________��
��4����ͼ��ʾ��װ������CH4��O2��KOH��Һ��ɵ�����ȼ�ϵ�أ����ø�װ�ÿ��Խ� ��ת��Ϊ �ܡ�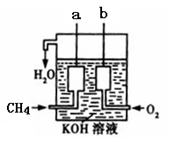
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���ս̰���л�ѧѡ��2 2.1���ĺϳ���ϰ���������棩 ���ͣ������
�ڰ��Ĺ�ҵ�ϳ��У��������ؼ��ļ������⣬������ѧ֪ʶ������ǡ�
(1)�ϳɰ��Ļ�ѧԭ����N2��3H2??2NH3���ڹ�ҵ��ѡ��20��50 MPa�Ľϸ�ѹǿ��450 �����ҵ��¶������з�Ӧ�������йصĻ�ѧԭ�����͡�
(2)��������������Ҫ�����������������¼��ֻ�������ķ���������ѡ�������˵ģ���˵�����ɡ�
A�����ˮ��ȡ����
B����ú��̿����ȡ������ú��̿�����ȣ����ȵ�̿��ˮ������Ӧ��������
C������Ȼ����������ȡ��������Ȼ������Ҫ�ɷּ����ڸ��¡���������������ˮ������Ӧ����������һ����̼��һ����̼�ڴ������ڵ���������ˮ������Ӧ�õ������Ͷ�����̼
(3)�ϳɰ��������������̣��������ʵ��ķ����������ϳɰ���ԭ�������������������������������Ļ�������ںϳ�������һ�������£�ͨ�����������ò�����������ͨ�������������ϵذ����ɵİ����������ͬʱδת���ķ�Ӧ�����ٽ���ϳ���ѭ���ӹ�������Ǻϳɰ��Ĺ������̡��������һ�������̻������̷���ͼ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com