
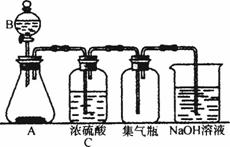
ͼ9-4
(1)��������ø�װ����ȡ������ռ������β���������������ϵ�Ҫ��
�ٶԷ�Ӧ���Ҫ��_____________________________________________________��
�ڶԷ�Ӧ������Ҫ��_____________________________________________________��
�۶������������ʵ�Ҫ��__________________________________________________��
(2)���ø�װ����ȡH2Sʱ��A����FeS2��B����ϡ���ᣬ��Ӧ����Һ���е���ɫ�Ļ��ǡ���
��ͼ9-4��ʾװ��Ӧ�����ı䶯��__________________________________��
��A�з�Ӧ�����ӷ���ʽΪ__________________________________��
��NaOH��Һ�з�Ӧ�����ӷ���ʽΪ__________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
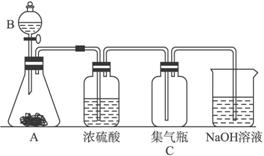
��1����������ø�װ����ȡ������ռ������յ�����Է�Ӧ���Ӧ�������������ʱ�����ϵ�Ҫ��
�ٶԷ�Ӧ���Ҫ��______________________________________________________��
�ڶԷ�Ӧ������Ҫ��____________________________________________________��
�۶������������ʵ�Ҫ��_________________________________________________��
��2���ø�װ����ȡ����ʱ��A���Ǹ�����أ�B����Ũ���ᣬ��Ӧ����Һ�д��ڴ���Mn2+���ֱ�д��A�к�NaOH��Һ��Ӧ�����ӷ���ʽ��
A��_________________________��NaOH��Һ��______________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com