
(14��)(1)Na2SO3��Һ���ɵõ��Ĺ���������___________��ԭ����______________.
(2)̼���Ⱶ��Һ���ɵõ��Ĺ���������___________��ԭ����___________________.
(3)����������Ũ�ȸ�Ϊ1mol/L �Ļ����10mL������Ũ����1mL�����õ�����ҺŨ��
Ϊ_________��ԭ����_____________________________________________________��
(4)��c(H+)��c(OH-)=kw���ƣ�FeS������Һ�д��ڣ�FeS(s) Fe
![]()
![]() +S
+S![]()
![]() ��
��
c(Fe![]() )��c(s
)��c(s )=ksp��������ksp=8.1��10-17������Һ�ܶ�Ϊ1.0g/cm3����
)=ksp��������ksp=8.1��10-17������Һ�ܶ�Ϊ1.0g/cm3����
������FeS���ܽ��Ϊ___________________��
 ��ĩ1�����ʽ���������ϵ�д�
��ĩ1�����ʽ���������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ÿ��2�֣���14�֣�A��B��C��D��Ϊ�������Ļ��������Է�������B��A��C����֪�л���A�Ľṹ��ʽΪ�� 
(1)A���ܺ�NaOH��Һ��Ӧ�����ܺ�NaHCO3��Һ��Ӧ��д��A�� NaHCO3��Һ��Ӧ�Ļ�ѧ����ʽ______ __________��
(2)���л���B��Ũ������������£����������һ�ֻ�״��(����ͼ)���л���B�Ľṹ��ʽΪ _��
�ڵ����ʵ���B��Na��NaOH��NaHCO3��ַ�Ӧ������Na��NaOH��NaHCO3�����ʵ���֮��Ϊ___ _____��
(3) 1molA��C��ȫȼ�գ�����O2���������,��1molC�ܺ�1molNa��ȫ��Ӧ��д����̼ԭ�������ٵ�C�Ľṹ��ʽ_____ ___��д��C��Ũ��ˮ�ķ�Ӧ�Ļ�ѧ����ʽ ��
(4)D��B��Ϊͬ���칹�壬��֪�䱽������������ȡ�����������ϵ�һ�ȴ���ֻ�����֣�D����NaHCO3��Һ��Ӧ������Na��NaOH��Ӧ����������D����Na��NaOH�����ʵ���֮��Ϊ2��3����D�Ľṹ��ʽΪ �� ��(ֻд����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(14��)��1��448mLij�����ڱ�״���µ�����Ϊ1.28g���������Ħ������ԼΪ
��2��12.4gNa2R�к�Na+0.4mol����Na2R��Ħ������Ϊ ,R�����ԭ������Ϊ
��3��5molCO2��������_________���ڱ�״������ռ�����ԼΪ__________�������ķ�����ĿԼΪ____________________��������ԭ�ӵ���ĿԼΪ___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012������ʡ��ˮһ�и���9�·��¿������ۺ����⣨��ѧ���֣� ���ͣ������
(14��)
��.��ÿ��1�֣� ��֪ij��ҵ��ˮ�к��д�����CuSO4��������Ag+��Hg2+�Լ��������࣬ͨ���������̿ɴӸ÷�ˮ�л�������ͭ���弰�������ʡ�
��1������1���õ��IJ����������ձ����� ��
��2������2�������ij���Լ����ٽ��������룬���Լ��ǣ��ѧʽ�� ����������ijɷ��ǣ��ѧʽ�� ��
��3������3���漰�IJ����ǣ�����Ũ���� �����ˡ���ɡ�
��4������2Ӧ����ͨ����н��У�ԭ���� ��
��.��(2)Ϊ1�֣�����ÿ��2�֣�ij��Һ�к���Na����SO��SO��CO��Cl����Br���е������֣����ν�������ʵ�飬�۲쵽�������¼���£��ټ������ᣬ����ɫ�������ɣ�����ԭ��Һ�еμ���ˮ��������������ټ�CCl4�����ã�CCl4��ʳ�ɫ���÷�Һ©����Һ�������Һ�����õ�ˮ��Һ�м���Ba(NO3)2��HNO3�Ļ����Һ���а�ɫ�������ɣ����ˣ�������Һ�м�AgNO3��HNO3�Ļ��Һ���а�ɫ��������.�Իش𣺣���ʾ������������SOΪSO��
(1)ԭ��Һ�п϶����ڵ����������� �������϶�û�е���������������
(2)������۸���BaCl2������Ļ����Һ�����ж�(1)��������Ӱ���� ��
(3)������з�����Ӧ�����ӷ���ʽΪ ���������� ��������.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ������ʡ����һ�и�һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
(14��)(I)�������Ӿ�Ϊ��ѧ��ѧ���������ӣ�Na����Ba2����Mg2����Al3����Fe3����Cu2����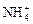 ��H����Cl����
��H����Cl����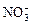 ��
��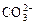 ��
��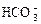 ��
��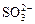 ��OH�������������У���ǿ������Һ�ﲻ���ܴ������ڵ���____________����ǿ������Һ�в����ܴ������ڵ�������____________���Ȳ�����ǿ������Һ��������ڣ�Ҳ������ǿ������Һ��������ڵ���______��������ǿ������Һ����ǿ������Һ������Դ������ڵ���____________
��OH�������������У���ǿ������Һ�ﲻ���ܴ������ڵ���____________����ǿ������Һ�в����ܴ������ڵ�������____________���Ȳ�����ǿ������Һ��������ڣ�Ҳ������ǿ������Һ��������ڵ���______��������ǿ������Һ����ǿ������Һ������Դ������ڵ���____________
(II)���ʵ������������±ߵĺ�����
(1)Cu2����______��Fe2����______��
(2) ______��H����______��H2O��CO2����
(3) 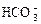 ��______��
��______��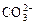 ��______��
��______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ������ʡ����9�·��¿������ۺ����⣨��ѧ���֣� ���ͣ������
(14��)
��.��ÿ��1�֣� ��֪ij��ҵ��ˮ�к��д�����CuSO4��������Ag+��Hg2+�Լ��������࣬ͨ���������̿ɴӸ÷�ˮ�л�������ͭ���弰�������ʡ�

��1������1���õ��IJ����������ձ����� ��
��2������2�������ij���Լ����ٽ��������룬���Լ��ǣ��ѧʽ�� ����������ijɷ��ǣ��ѧʽ�� ��
��3������3���漰�IJ����ǣ�����Ũ���� �����ˡ���ɡ�
��4������2Ӧ����ͨ����н��У�ԭ���� ��
��.��(2)Ϊ1�֣�����ÿ��2�֣�ij��Һ�к���Na����SO��SO��CO��Cl����Br���е������֣����ν�������ʵ�飬�۲쵽�������¼���£��ټ������ᣬ����ɫ�������ɣ�����ԭ��Һ�еμ���ˮ��������������ټ�CCl4�����ã�CCl4��ʳ�ɫ���÷�Һ©����Һ�������Һ�����õ�ˮ��Һ�м���Ba(NO3)2��HNO3�Ļ����Һ���а�ɫ�������ɣ����ˣ�������Һ�м�AgNO3��HNO3�Ļ��Һ���а�ɫ��������.�Իش𣺣���ʾ������������SOΪSO��
(1)ԭ��Һ�п϶����ڵ����������� �������϶�û�е���������������
(2)������۸���BaCl2������Ļ����Һ�����ж�(1)��������Ӱ���� ��
(3)������з�����Ӧ�����ӷ���ʽΪ ���������� ��������.
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com