��X��Y��Z��W���ֶ�����Ԫ�أ�ԭ����������������˵�����ܺ�Ϊ38��YԪ��ԭ������������ռ�����ܵ�������
��WԪ��ԭ��������������ͬ����ZԪ��ԭ��������������5�����ӣ�W�� Y������ͬһ���壮
��1������Yԭ�ӽṹʾ��ͼ��
��
��2���ӻ��ϼ۽Ƕȷ�����XԪ�ؿ����������ڱ���IA��VIIA��
IVA
IVA
�壻
��3��Z��W����Ԫ������������Ӧˮ���������Ӧ�Ļ�ѧ����ʽ
Mg��OH��2+2HClO4=Mg��ClO4��2+2H2O
Mg��OH��2+2HClO4=Mg��ClO4��2+2H2O
��
��4����֪X
2���ʵ���ֵΪ143kJ?g
-1�����X
2������Y
2������ȼ�յ��Ȼ�ѧ����ʽ��
H
2��g��+
O
2��g��=H
2O��1������H=-286kJ?mol
-1H
2��g��+
O
2��g��=H
2O��1������H=-286kJ?mol
-1��



 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��


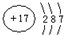
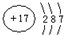


 ��X��Y��Z��W���ֶ�����Ԫ�أ�ԭ��������������X�������Ӿ���һ�����ӣ�Z��W�����ڱ��ڴ���ͬһ��������λ�ã����ǵĵ�����ͨ��״���¾�Ϊ��ɫ���壮Yԭ�ӵ������������Ǵ�����������2������ش�
��X��Y��Z��W���ֶ�����Ԫ�أ�ԭ��������������X�������Ӿ���һ�����ӣ�Z��W�����ڱ��ڴ���ͬһ��������λ�ã����ǵĵ�����ͨ��״���¾�Ϊ��ɫ���壮Yԭ�ӵ������������Ǵ�����������2������ش�