
�����£���1mol��CuSO4��5H2O��s������ˮ��ʹ��Һ�¶Ƚ��ͣ���ЧӦΪ��H1����1mol��CuSO4��s������ˮ��ʹ��Һ�¶����ߣ���ЧӦΪ��H2��CuSO4��5H2O���ȷֽ�Ļ�ѧ����ʽΪ��CuSO4��5H2O��s�� CuSO4��s��+5H2O��1������ЧӦΪ��H3���������ж���ȷ����
CuSO4��s��+5H2O��1������ЧӦΪ��H3���������ж���ȷ����
A����H2>��H3 B����H1+��H3=��H2
C����H1<��H3 D����H1+��H2=��H3
 ����ʦ���һ��һ��ϵ�д�
����ʦ���һ��һ��ϵ�д� �Ͻ�ƽ��У����ϵ�д�
�Ͻ�ƽ��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016�����ʡ������ѧ�ڵڶ����¿����ۻ�ѧ�Ծ��������棩 ���ͣ������
��14�֣���ҵ�Ͽ�����Ȼ��Ϊԭ���Ʊ��״���Ҳ����ˮú���ϳɼ״���
��1����֪2CH4��g����O2��g��=2CO2��g����4H2��g�� ��H��akJ/mol
CO��g����2H2��g����CH3OH��g����H��bkJ/mol
��д����CH4��O2��ȡ������Ȼ�ѧ����ʽ��___________________��
��2��������ͨ�����з�Ӧ�Ʊ��״���CO��g����2H2��g�� CH3OH��g����ͼ���Ƿ�ӦʱCO��g����CH3OH��g����Ũ����ʱ��t�ı仯������ӷ�Ӧ��ʼ��ƽ�⣬��CO��ʾƽ����Ӧ����v��CO����____________���÷�Ӧ��ƽ�ⳣ������ʽΪ______________��
CH3OH��g����ͼ���Ƿ�ӦʱCO��g����CH3OH��g����Ũ����ʱ��t�ı仯������ӷ�Ӧ��ʼ��ƽ�⣬��CO��ʾƽ����Ӧ����v��CO����____________���÷�Ӧ��ƽ�ⳣ������ʽΪ______________��
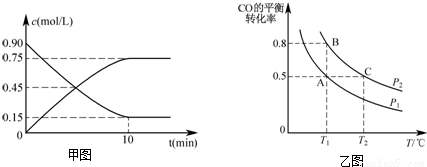
��3����һ�ݻ��ɱ���ܱ������г���10mol CO��20mol H2��CO��ƽ��ת�������¶ȣ�T����ѹǿ��P���ı仯����ͼ��ʾ��
������˵�������жϸ÷�Ӧ�ﵽ��ѧƽ��״̬����___________��������ĸ��
A��H2���������ʵ���CH3OH���������ʵ�2��
B��H2������������ٸı�
C����ϵ��H2��ת���ʺ�CO��ת�������
D����ϵ�������ƽ��Ħ���������ٸı�
�ڱȽ�A��B����ѹǿ��СPA___________PB�����������=������
�����ﵽ��ѧƽ��״̬Aʱ�����������Ϊ20L�������Ӧ��ʼʱ�Գ���10molCO��20molH2������ƽ��״̬Bʱ���������V��B��=___________L��
��4���Լ״�Ϊȼ�ϣ�����Ϊ��������KOH��ҺΪ�������Һ�����Ƴ�ȼ�ϵ�أ��缫����Ϊ���Ե缫����
����KOH��Һ���������ظ�����Ӧ�����ӷ���ʽΪ_____________��
�����������Һ��KOH�����ʵ���Ϊ1��0mol������0��75mol�״����뷴Ӧʱ���������Һ�и������ӵ����ʵ���Ũ���ɴ�С��˳����______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016���㽭ʡ������ѧ��10���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
X��Y��Z��W����ͼ��ʾ��ת����ϵ����X��Y������
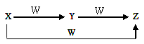
��C��CO ��S��SO2 ��AlCl3��Al��OH��3 ��Cl2��FeCl3
A�����Т٢� B���٢ڢ� C�����Тڢ� D���٢ڢۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�����ɹŰ�������һ��10���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
����������ȷ����
A��1 mol H2O������Ϊ18g/mol
B��CH4��Ħ������Ϊ16g
C��3��01��1023��SO2���ӵ�����Ϊ32g
D����״���£�1 mol�κ����������Ϊ22��4L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��ӱ�ʡ�߶���ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��ͼ����ͬ��������H2O2�ֽ�ĶԱ�ʵ��ʱ���ų�O2�������ʱ��ı仯��ϵʾ��ͼ��aΪʹ�ô�����bΪ��ʹ�ô�����������ȷ��ͼ����
A�� B��
B��
C��  D��
D��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�꼪��ʡ�߶���ѧ��9���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
������������Ӧ����ʽ
H2��g����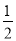 O2��g����H2O��g�� ��H��a kJ/mol
O2��g����H2O��g�� ��H��a kJ/mol
H2��g����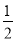 O2��g����H2O�� l �� ��H��b kJ/mol
O2��g����H2O�� l �� ��H��b kJ/mol
2H2��g����O2��g����2H2O�� l �� ��H��c kJ/mol
�������ǵ�����������ȷ����
A�����Ƕ������ȷ�Ӧ B��a��b��c��Ϊ��ֵ
C��a��b D��2b��c
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�츣��ʡ�����и�����ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�����г�������������ˮ������ijѧ��������ˮ�����������ʵ���Һ�������������ҩƷ���ʵ���
A��ʯ�� B�������� C���������� D���Ȼ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ������ʵ����ѧ��һ��10�½β⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
���и��������У�������������ͬ����
A��10g H2��10g O2 B��5��6L N2����״������22g CO2
C��9g H2O��0��5mol Br2 D��224ml H2����״������0��lmol N2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016������ʡ����10���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�������ӷ���ʽ����д�����۾���������
ѡ�� | ���ӷ���ʽ | ���� |
A | ��1 mol Cl2ͨ�뵽��1 mol FeI2��Һ�У� 2Fe2����2I����2Cl2===2Fe3����4Cl����I2 | ��ȷ��Cl2�������ɽ�Fe2����I�������� |
B | Mg��HCO3��2��Һ��������NaOH��Һ��Ӧ�� Mg2����HCO3-��OH��===MgCO3����H2O | ��ȷ����ʽ����Ӧ�������κ�ˮ |
C | ����SO2ͨ�뵽NaClO��Һ�У� SO2��H2O��ClO��===HClO��HSO3- | ��ȷ��˵�����ԣ� H2SO3ǿ��HClO |
D | 1 mol��L��1��NaAlO2��Һ��2��5 mol��L��1��HCl��Һ�����������Ȼ�ϣ�2AlO2-��5H��===Al3����Al��OH��3����H2O | ��ȷ��AlO2-��Al��OH��3���ĵ�H�������ʵ���֮��Ϊ2��3 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com