
�ڱ�״���£�ij��̬��A���ܶ�Ϊ1.25 g��L��1��A��һ���������ܷ�����ͼ��ʾ�ı仯�����е��������ˮ�ȣ���ʡ�ԣ���֪2CH3CHO��O2![]() 2CH3COOH��M����(g��cm��3)��22.4(L��mol��1)��
2CH3COOH��M����(g��cm��3)��22.4(L��mol��1)��
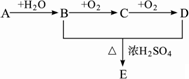
������ͼ�仯��ϵ������������⣺
(1)�ƶ�A��B��C��D��E�Ľṹ��ʽ��
A��________��B��________��C��________��D��________��E��________��
(2)д�����з�Ӧ�Ļ�ѧ����ʽ����ָ�����ں����л���Ӧ���ͣ�
A��B��________��________��
B��D��E��________��________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ����Ʊ�����ѧ�˽̰� �˽̰� ���ͣ�038
�ڱ�״���£�ij��̬�����ܶ���1.34 g��L��1����0.1 mol��������ȼ������8.8 g��CO2��5.4 g��H2O����ȷ��������ķ���ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

������ͼ�仯��ϵ,����������⣺
(1)�ƶ�A��B��C��D��E�Ľṹ��ʽ��
A.____________��B._____________��C.____________��D.______________,E.__________��
(2)д�����з�Ӧ�Ļ�ѧ����ʽ����ָ�����ں����л���Ӧ���ͣ�
A��B��_____________________________________________��________________��
B+D��E��___________________________________________��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ���� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ڱ�״���£�ij��̬�л���4L��������Ϊ12.5g������л�������ʽ�����ǣ�
A CH B CH2 C CH3 D CH2O
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com