
(12��)��ѧ��ѧ�г����ļ������ʴ������¹�ϵ�����м��Ǻ�ɫ�ǽ������ʣ����������г����Ľ������ʣ�C�ڳ�����Ϊ��ɫ��Һ�壬D�Ǻ���ɫ���塣��ͼ�в��ֲ���ͷ�Ӧ��������ȥ��

�ش��������⣺
(1)д������A��Ũ��Һ��Ӧ�Ļ�ѧ����ʽ_________________________________��
(2)C�ĵ���ʽ��___________________________��
(3)��ȥG�����к���H���ʲ��õķ�����_____________________��
(4)A��Һ��һ����ʹʪ��ĺ�ɫʯ����ֽ���������巴Ӧ������һ���Σ����ε���Һ�����ԣ���ԭ���ǣ������ӷ���ʽ��ʾ��_____________________________��
(5)д����Fת��ΪE�����ӷ���ʽ____________________________________��
(6)д����Gת��ΪH�����ӷ���ʽ____________________________________��
��12�֣���1��C+4HNO3��Ũ��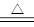 CO2��+4NO2��+2H2O ��2�֣�
CO2��+4NO2��+2H2O ��2�֣�
��2��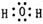 ��2�֣�
��3�����ȣ�2�֣�
��2�֣�
��3�����ȣ�2�֣�
��4��NH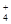 + H2O
+ H2O NH3��H2O + H+ ��2�֣�
NH3��H2O + H+ ��2�֣�
��5��2Fe3+ ��Fe 3Fe2+��2�֣�
3Fe2+��2�֣�
��6��CO32- +H2O +CO2=2HCO3-��2�֣�
���������ۺϸ����ʵ���ɫ״̬�������ʵ�ת����ϵ�����ж���Ϊ̼��AΪ���
C+4HNO3��Ũ��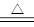 CO2��+4NO2��+2H2O ��2�֣����е�DΪNO2��CΪ��2�ϡ�BΪCO2��
CO2��+4NO2��+2H2O ��2�֣����е�DΪNO2��CΪ��2�ϡ�BΪCO2��
����ͽ����ҵIJ������ҵ����Ƕ����йأ���֪��Ϊ��۽������ɲ¶�Ϊ����


 ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12 �֣���ÿ��2�֣�CH3COOH����ѧ��ѧ�г��õ�һԪ���ᣬ��ش��������⣺
��1�����ֱ�pH=2������ʹ���ϡ��100������ϡ�ͺ���Һ��pH������ ���ᣨ���������������������
��2����100mL0.1mol��L-1��CH3COOH��Һ��50mL 0.2mol��L-1��NaOH��Һ��ϣ�������Һ�� �ԣ�ԭ�� �������ӷ���ʽ��ʾ����
��3����֪ij�����Һ��ֻ����CH3COO-��H+��Na+��OH-�������ӣ�������Ũ�ȴ�С��ϵΪ��c(CH3COO-)>c(H+)> c(Na+)> c(OH-)�������Һ�к��е�����Ϊ ��
��4����֪Ka(CH3COOH)= 1.76��10-5��Ka(HNO2)= 4.6��10-4������ͬŨ�ȵ�NaOH��Һ�ֱ��к͵������pH��ȵ�CH3COOH��HNO2��������NaOH��Һ�������ϵΪ��
ǰ�� ���ߣ��>��<��=����
��5����֪25��ʱ��0.1mol��L-1������Һ��pHԼΪ3�������м������������ƾ��壬������Һ��pH�����������������ֲ�ͬ�Ľ��ͣ���ͬѧ��Ϊ�����Ƴʼ��ԣ�������Һ��pH������ͬѧ��������һ�ֲ�ͬ�ڼ�ͬѧ�Ľ��ͣ�����д����ͬѧ���ܵ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ɹŰ�������һ�и���9���¿���ѧ�Ծ����������� ���ͣ������
(12��)A��B��C��D��Ϊ��ѧ��ѧ�г��������ʣ�����֮���ת����ϵ����ͼ(���ֲ�������ȥ)��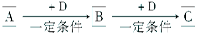
�Իش�
��1����D�Ǿ��������Եĵ��ʣ�AԪ�������������Ԫ�أ���AΪ________(��Ԫ�ط���)��
��2����D�ǽ�����C��Һ�ڴ���ʱӦ��������D����������(�ñ�Ҫ�����ֺ����ӷ���ʽ��ʾ)____________________________________________________��D�ڳ�ʪ�Ŀ���������������ʴ��д����ʴʱԭ��������ĵ缫��Ӧʽ�� ��
��3����A��B��CΪ��ͬһ�ֽ���Ԫ�ص������������Һ��A��C��Ӧ����B����д��Bת��ΪC�����п��ܵ����ӷ���ʽ��
______________________________��_________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����������ѧ����8���¿���ѧ�Ծ����������� ���ͣ������
(12��)��ѧ��ѧ�г����ļ������ʴ������¹�ϵ�����м��Ǻ�ɫ�ǽ������ʣ����������г����Ľ������ʣ�C�ڳ�����Ϊ��ɫ��Һ�壬D�Ǻ���ɫ���塣��ͼ�в��ֲ���ͷ�Ӧ��������ȥ��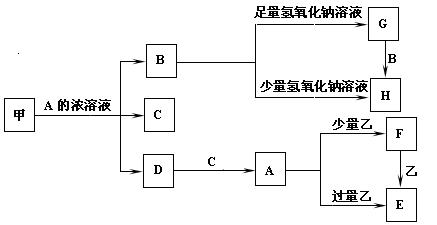
�ش��������⣺
(1)д������A��Ũ��Һ��Ӧ�Ļ�ѧ����ʽ_________________________________��
(2)C�ĵ���ʽ��___________________________��
(3)��ȥG�����к���H���ʲ��õķ�����_____________________��
(4)A��Һ��һ����ʹʪ��ĺ�ɫʯ����ֽ���������巴Ӧ������һ���Σ����ε���Һ�����ԣ���ԭ���ǣ������ӷ���ʽ��ʾ��_____________________________��
(5)д����Fת��ΪE�����ӷ���ʽ____________________________________��
(6)д����Gת��ΪH�����ӷ���ʽ____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���㽭ʡ�����и�����ѧ��������У������ѧ�Ծ����������� ���ͣ������
(12��)��ͼ�У�A��LΪ�������ʻ�����ʵ�ˮ��Һ��B��A������ȼ�ղ�����ɫ�̣�B��GΪ��ѧ��ѧ�г����������ʣ�I����ɫ��ӦΪ��ɫ�����J��Ԫ��ԭ�Ӻ���ֻ��һ�����ӣ�FΪ��ɫ���д̼�����ζ���壬����ʹƷ����Һ��ɫ��
�ش��������⣺
(1)K�����Ļ�ѧ���� ��
(2)D��ˮ��Һ��G��Ӧ�������ӷ���ʽΪ_______________________________________________��
(3)д����ҵ����ȡ����G�Ļ�ѧ����ʽ ��
(4)������Fͨ������װ����
д��A��B�е����ӷ���ʽ��
�� ��
(5)����Fͨ��һ����K��ˮ��Һ�У���������Һ�и�����Ũ��һ������Ĺ�ϵʽΪ
��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com