
(12��)����Ҫ��ش�������⣺
(1) ��ȥ���������������е���������(������Ϊ����)��д���йصķ�Ӧ����ʽ��
��ͭ�� (����) ��
��FeCl3 ��Һ(FeCl2 ) ��
��N2 (O2) ____________________________________________________��
(2)��ͼ��ʾ����仯��δ֪���о�������Ԫ�أ�EΪ����ɫ��ĩ���ݴ˻ش��������⣺

��д��A��B�Ļ�ѧ����ʽ���������ת�Ƶķ������Ŀ��
____________________________________________________________
����B��C�ı仯������C����Һ�������������е����ʣ�������ˮ�������� ����Ҫԭ���� ���������� ����Ҫԭ���� ��
(1) ��2Al + 2H2O + 2OH- = AlO2- + 3H2 ���� 2Al + 6H+ == 2Al3+ + 3H2��
�� 2FeCl2
+ Cl2 ==3 FeCl3
�� 2Cu + O2  2CuO
2CuO
(2) ��������NaOH ����CO2 ���٣�NaOH����;������NaHCO3 ����CO2 ������Na2CO3ת��ΪNaHCO3
����������1����������������������Һ�������У���ͭ���ܣ��ݴ˿��Գ�ȥ��������ʽΪ2Al + 2H2O + 2OH- = AlO2- + 3H2 ���� 2Al + 6H+ == 2Al3+ + 3H2����
���Ȼ��������л�ԭ�ԣ��ܱ��������������Ȼ��������Գ�ȥ�Ȼ����е��Ȼ���������������������ʽΪ2FeCl2 + Cl2 ==3 FeCl3��
�������ܺ�ͭ��Ӧ�����������ܣ�����Ҫ��ȥ�����е�����������ͨ�����ȵ�ͭ������ʽΪ2Cu + O2  2CuO ��
2CuO ��
��2��EΪ����ɫ��ĩ���Һ�����Ԫ�أ�����E�ǹ������ơ���A���ƣ�B���������ƣ�C��̼���ƣ�F��̼�����ƣ�D���Ȼ��ơ�
���ƺ�ˮ�ķ�Ӧ�У���ʧȥ���ӣ�����ԭ����ˮ���������õ����ӣ�����ʽΪ��
��CO2���������Ʒ�Ӧ�У�����������ƹ�����������̼�����л�����������ƣ����CO2��������̼�����ܺ�CO2��Ӧ����̼�����ƣ��������ʿ�����̼�����ơ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(12��) ����Ҫ������������⣺
��1�������������ʵ��������ʣ��ж��侧�����ͣ�
A����̬ʱ�ܵ��磬���������B��������CS2��������ˮ��C����̬��Һ̬ʱ�������磬�۵�3500��
A�� B�� C��
��2��ָ�������K3[Co(CN)6]�е��������ӡ����弰����λ����_________��__________��_________��
(3)��H2��SiC��CO2��HF�У��ɼ��Լ���ɵķǼ��Է����� ���ɷǼ��Լ��γɵķǼ��Է����� �����γɷ��Ӿ���Ļ������� ����������ľ���Ļ�ѧʽ ������ԭ�Ӿ������ �����������۵��ɸߵ��͵�˳���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��㶫ʡ������������ѧ�߶���ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
(7��)����Ҫ��ش���������
(1)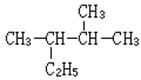 ________________________________ ��д���ƣ�
________________________________ ��д���ƣ�
��2��2������2����ϩ ��д�ṹ��ʽ��
��3������ʽ ��ʾ�ķ���ʽ ��
��ʾ�ķ���ʽ ��
(4)�������ŵIJ�ͬ�����л�����з��࣬��ָ�������л�������࣬���ں����ϡ�
CH3CH2CH2Br __________�� ___________��
___________��  ________________��
________________�� ________________��
________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�갲��ʡ�������и�һ��ѧ����ĩ���Ի�ѧA������������ ���ͣ������
(12��)����Ҫ��ش�������⣺
(1) ��ȥ���������������е���������(������Ϊ����)��д���йصķ�Ӧ����ʽ��
��ͭ�� (����) ��
��FeCl3��Һ(FeCl2 ) ��
��N2 (O2) ____________________________________________________��
(2)��ͼ��ʾ����仯��δ֪���о�������Ԫ�أ�EΪ����ɫ��ĩ���ݴ˻ش��������⣺ 
��д��A��B�Ļ�ѧ����ʽ���������ת�Ƶķ������Ŀ��
____________________________________________________________
����B��C�ı仯������C����Һ�������������е����ʣ�������ˮ�������� ����Ҫԭ���� ���������� ����Ҫԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011--2012ѧ���Ĵ�ʡ�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
(12��) ����Ҫ������������⣺
��1�������������ʵ��������ʣ��ж��侧�����ͣ�
A����̬ʱ�ܵ��磬���������B��������CS2��������ˮ��C����̬��Һ̬ʱ�������磬�۵�3500��
A�� B�� C��
��2��ָ�������K3[Co(CN)6]�е��������ӡ����弰����λ����_________��__________��_________��
(3)��H2��SiC��CO2��HF�У��ɼ��Լ���ɵķǼ��Է����� ���ɷǼ��Լ��γɵķǼ��Է����� �����γɷ��Ӿ���Ļ������� ����������ľ���Ļ�ѧʽ ������ԭ�Ӿ������ �����������۵��ɸߵ��͵�˳���� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com